Avocado Oil vs Olive Oil – Which is Healthier?
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
Our Verdict
When comparing avocado oil to olive oil, we picked the olive oil.
Why?
Avocados and olives are both very healthy foods. However, when they are made into oils, there’s an important distinguishing factor:
Olive oil usually retains a lot of the micronutrients from the olives (including vitamins E and K), whereas no measurable micronutrients usually remain in avocado oil.
So while both olive oil and avocado oil have a similar (excellent; very heart-healthy!) lipids profile, the olive oil has some bonuses that the avocado oil doesn’t.
We haven’t written about the nutritional profiles of either avocados or olives yet, but here’s what we had to say on the different kinds of olive oil available:
And here’s an example of a good one on Amazon, for your convenience 😎
Don’t Forget…
Did you arrive here from our newsletter? Don’t forget to return to the email to continue learning!
Recommended
Learn to Age Gracefully
Join the 98k+ American women taking control of their health & aging with our 100% free (and fun!) daily emails:
-
We Hope This Email Blows Your Tits Clean Off
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
The Right Kind Of “Email Hacks”!
Are you a Gmailer or an Outlookista? Whatever your preference, you’re probably facing many of the same challenges that most of us face in our work and personal lives:
Email’s greatest strength (its ease of accessibility) brings about its greatest problem (our inboxes are cluttered and chaotic), not to mention that each of us are usually managing a whole flock of email addresses.
Sometimes we put productivity resources up against each other; that’s not what we’re going to do today! Each of these can play a role alongside each other; grab as many as will make your life easier:
ProtonMail: this is an email client; it’s the nicest, simplest, easiest, free email client that doesn’t track, let alone share, everything you do.
Bonus: there also exists ProtonCalendar (it’s a calendar that doesn’t share your data), ProtonDrive (it’s a cloud storage provider that doesn’t share your data) and, because they’re indeed serious about your privacy, ProtonVPN (it’s a VPN that, of course, doesn’t share your data).
Clean Email: maybe you’re stuck with the email provider you have. It happens. But it doesn’t have to be a chaotic mess. This tool will make tidying your email (and keeping it tidy!) a simplified dream.
See How Clean Your Email Can Get With Just A Few Clicks!
Right Inbox: a Gmail extension with many useful features, including read receipts, emails scheduled for later (e.g: time your email to send at 7am to look like a morning lark when in fact you’re peacefully snoozing), add unforwardable “For Your Eyes Only” notes to emails, and more.
Power Up Your Gmail With The Right Inbox Extension!
Email Finder: find the verified work email address of any person, so long as you know what company you’re looking for them in! No more “I thought it was lastname.firstname@ and it was firstname.lastname@”, no more “the wrong John Smith”, no more “undelivered” bounceback notices. Just: your email delivered.
Never Hear From The Mailer Daemon Again, With Email Finder!
Unroll.me: love your subscriptions, but hate the clutter? Unroll.me aggregates them for you in a virtual roll-up, with an “unroll” button to read them.
Get What You Really Want From Your Subscriptions, With Unroll.Me!
On which note, anything you’d like to hear more of from us? Let us know! You can always just hit reply, or use the feedback widget at the bottom of this email
Share This Post
-
Lime-Charred Cauliflower Popcorn
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
Called “popcorn” for its appearance and tasty-snackness, this one otherwise bears little relation to the usual movie theater snack, and it’s both tastier and healthier. All that said, it can be eaten on its own as a snack (even with a movie, if you so wish), or served as one part of a many-dish banquet, or (this writer’s favorite) as a delicious appetizer that also puts down a healthy bed of fiber ready for the main course to follow it.
You will need
- 1 cauliflower, cut into small (popcorn-sized) florets
- 2 tbsp extra virgin olive oil
- 1 tbsp lime pickle
- 1 tsp cumin seeds
- 1 tsp smoked paprika
- 1 tsp chili flakes
- 1 tsp black pepper, coarse ground
- ½ tsp ground turmeric
Method
(we suggest you read everything at least once before doing anything)
1) Preheat your oven as hot as it will go
2) Mix all the ingredients in a small bowl except the cauliflower, to form a marinade
3) Drizzle the marinade over the cauliflower in a larger bowl (i.e. big enough for the cauliflower), and mix well until the cauliflower is entirely, or at least almost entirely, coated. Yes, it’s not a lot of marinade but unless you picked a truly huge cauliflower, the proportions we gave will be enough, and you want the end result to be crisp, not dripping.
4) Spread the marinaded cauliflower florets out on a baking tray lined with baking paper. Put it in the oven on the middle shelf, so it doesn’t cook unevenly, but keeping the temperature as high as it goes.
5) When it is charred and crispy golden, it’s done—this should take about 20 minutes, but we’ll say ±5 minutes depending on your oven, so do check on it periodically—and time to serve (it is best enjoyed warm).
Enjoy!
Want to learn more?
For those interested in some of the science of what we have going on today:
- We must do a main feature on the merits of cruciferous vegetables! Watch this space.
- All About Olive Oils (Extra Virgin & Otherwise)
- Capsaicin For Weight Loss And Against Inflammation
- Black Pepper’s Impressive Anti-Cancer Arsenal (And More)
- Why Curcumin (Turmeric) Is Worth Its Weight In Gold
Take care!
Share This Post
-
Qigong: A Breath Of Fresh Air?
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
Qigong: Breathing Is Good (Magic Remains Unverified)
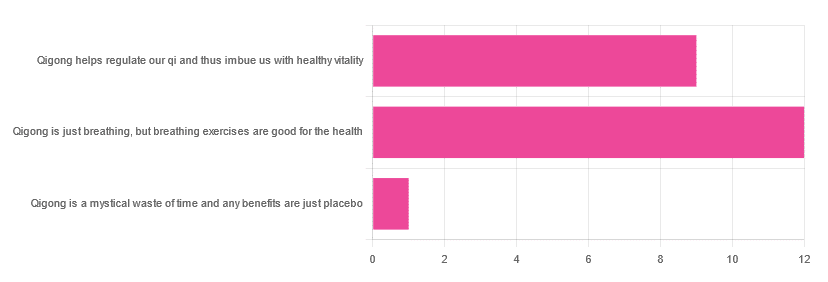
In Tuesday’s newsletter, we asked you for your opinions of qigong, and got the above-depicted, below-described, set of responses:
- About 55% said “Qigong is just breathing, but breathing exercises are good for the health”
- About 41% said “Qigong helps regulate our qi and thus imbue us with healthy vitality”
- One (1) person said “Qigong is a mystical waste of time and any benefits are just placebo”
The sample size was a little low for this one, but the results were quite clearly favorable, one way or another.
So what does the science say?
Qigong is just breathing: True or False?
True or False, depending on how we want to define it—because qigong ranges in its presentation from indeed “just breathing exercises”, to “breathing exercises with visualization” to “special breathing exercises with visualization that have to be exactly this way, with these hand and sometimes body movements also, which also must be just right”, to far more complex definitions that involve qi by various mystical definitions, and/or an appeal to a scientific analog of qi; often some kind of bioelectrical field or such.
There is, it must be said, no good quality evidence for the existence of qi.
Writer’s note, lest 41% of you want my head now: I’ve been practicing qigong and related arts for about 30 years and find such to be of great merit. This personal experience and understanding does not, however, change the state of affairs when it comes to the availability (or rather, the lack) of high quality clinical evidence to point to.
Which is not to say there is no clinical evidence, for example:
Acute Physiological and Psychological Effects of Qigong Exercise in Older Practitioners
…found that qigong indeed increased meridian electrical conductance!
Except… Electrical conductance is measured with galvanic skin responses, which increase with sweat. But don’t worry, to control for that, they asked participants to dry themselves with a towel. Unfortunately, this overlooks the fact that a) more sweat can come where that came from, because the body will continue until it is satisfied of adequate homeostasis, and b) drying oneself with a towel will remove the moisture better than it’ll remove the salts from the skin—bearing in mind that it’s mostly the salts, rather than the moisture itself, that improve the conductivity (pure distilled water does conduct electricity, but not very well).
In other words, this was shoddy methodology. How did it pass peer review? Well, here’s an insight into that journal’s peer review process…
❝The peer-review system of EBCAM is farcical: potential authors who send their submissions to EBCAM are invited to suggest their preferred reviewers who subsequently are almost invariably appointed to do the job. It goes without saying that such a system is prone to all sorts of serious failures; in fact, this is not peer-review at all, in my opinion, it is an unethical sham.❞
~ Dr. Edzard Ernst, a founding editor of EBCAM (he since left, and decries what has happened to it since)
One of the other key problems is: how does one test qigong against placebo?
Scientists have looked into this question, and their answers have thus far been unsatisfying, and generally to the tune of the true-but-unhelpful statement that “future research needs to be better”:
Problems of scientific methodology related to placebo control in Qigong studies: A systematic review
Most studies into qigong are interventional studies, that is to say, they measure people’s metrics (for example, blood pressure, heart rate, maybe immune function biomarkers, sleep quality metrics of various kinds, subjective reports of stress levels, physical biomarkers of stress levels, things like that), then do a course of qigong (perhaps 6 weeks, for example), then measure them again, and see if the course of qigong improved things.
This almost always results in an improvement when looking at the before-and-after, but it says nothing for whether the benefits were purely placebo.
We did find one study that claimed to be placebo-controlled:
…but upon reading the paper itself carefully, it turned out that while the experimental group did qigong, the control group did a reading exercise. Which is… Saying how well qigong performs vs reading (qigong did outperform reading, for the record), but nothing for how well it performs vs placebo, because reading isn’t a remotely credible placebo.
See also: Placebo Effect: Making Things Work Since… Well, A Very Long Time Ago ← this one explains a lot about how placebo effect does work
Qigong is a mystical waste of time: True or False?
False! This one we can answer easily. Interventional studies invariably find it does help, and the fact remains that even if placebo is its primary mechanism of action, it is of benefit and therefore not a waste of time.
Which is not to say that placebo is its only, or even necessarily primary, mechanism of action.
Even from a purely empirical evidence-based medicine point of view, qigong is at the very least breathing exercises plus (usually) some low-impact body movement. Those are already two things that can be looked at, mechanistic processes pointed to, and declarations confidently made of “this is an activity that’s beneficial for health”.
See for example:
- Effects of Qigong practice in office workers with chronic non-specific low back pain: A randomized control trial
- Qigong for the Prevention, Treatment, and Rehabilitation of COVID-19 Infection in Older Adults
- Impact of Medical Qigong on quality of life, fatigue, mood and inflammation in cancer patients: a randomized controlled trial
…and those are all from respectable journals with meaningful peer review processes.
None of them are placebo-controlled, because there is no real option of “and group B will only be tricked into believing they are doing deep breathing exercises with low-impact movements”; that’s impossible.
But! They each show how doing qigong reliably outperforms not doing qigong for various measurable metrics of health.
And, we chose examples with physical symptoms and where possible empirically measurable outcomes (such as COVID-19 infection levels, or inflammatory responses); there are reams of studies showings qigong improves purely subjective wellbeing—but the latter could probably be claimed for any enjoyable activity, whereas changes in inflammatory biomarkers, not such much.
In short: for most people, it indeed reliably helps with many things. And importantly, it has no particular risks associated with it, and it’s almost universally framed as a complementary therapy rather than an alternative therapy.
This is critical, because it means that whereas someone may hold off on taking evidence-based medicines while trying out (for example) homeopathy, few people are likely to hold off on other treatments while trying out qigong—since it’s being viewed as a helper rather than a Hail-Mary.
Want to read more about qigong?
Here’s the NIH’s National Center for Complementary and Integrative Health has to say. It cites a lot of poor quality science, but it does mention when the science it’s citing is of poor quality, and over all gives quite a rounded view:
Enjoy!
Share This Post
Related Posts
-
These Top Few Things Make The Biggest Difference To Health
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
The Best Few Interventions For The Best Health
Writer’s note: I was going to do something completely different for today (so that can go out another week now), but when reflecting on my own “what should I focus on in the new year?” (in terms of my own personal health goals and such) it occured to me that I should look back on the year’s articles, to take our own advice myself, and see what most important things I should make sure to focus on.
In so doing for myself, it occured to me that you, our subscribers who like condensed information and simple interventions for big positive effects, might also find value in a similar once-over. And so, today’s main feature was born!
Sometimes at 10almonds we talk about “those five things that affect everything”. They are:
- Good diet
- Good exercise
- Good sleep
- Not drinking
- Not smoking
If we were to add a sixth in terms of things that make a huge difference, it would be “manage stress effectively” and a seventh, beyond the scope of our newsletter, would be “don’t be socioeconomically disadvantaged” (e.g. poor, and/or part of some disprivileged minority group).
But as for those five we listed, it still leaves the question: what are the few most effective things we can do to improve them? Where can we invest our time/energy/effort for greatest effect?
Good diet
Best current science consistently recommends the Mediterranean Diet:
The Mediterranean Diet: What Is It Good For?
But it can be tweaked for specific desired health considerations:
Four Ways To Upgrade The Mediterranean Diet
Other most-effective dietary tweaks that impact a lot of other areas of health include looking after your gut health and looking after your blood sugars:
Making Friends With Your Gut (You Can Thank Us Later)
and
“Let Them Eat Cake”, She Said (10 Ways To Balance Blood Sugars)
Good exercise
Most exercise is good, but two of the most beneficial things that are (for most people) easy to implement are walking, and High-Intensity Interval Training:
How To Do HIIT (Without Wrecking Your Body)
Good sleep
This means quality and quantity! We cannot skimp on either and expect good health:
Why You Probably Need More Sleep
and as for quality,
The Head-To-Head Of Google and Apple’s Top Apps For Getting Your Head Down
Not drinking
According to the World Health Organization, the only safe amount of alcohol is zero.
See also:
Can We Drink To Good Health? (e.g. Red Wine & Heart Health)
and
Not smoking
We haven’t done a main feature on this! It’s probably not really necessary, as it’s not very contentious to say “smoking is bad for everything”.
WHO | Tobacco kills up to half its users who don’t quit
However, as a side-note, while cannabis is generally recognised as not as harmful as tobacco-based products, it has some fairly major drawbacks too. For some people, the benefits (e.g. pain relief) may outweigh the risks, though:
Final thoughts
Not sure where to start? We suggest this order of priorities, unless you have a major health condition that makes something else a higher priority:
- If you smoke, stop
- If you drink, reduce, or ideally stop
- Improve your diet
About that diet…
- Worry less about what to exclude, and instead focus on adding more variety of fruit/veg
- See also: Level-Up Your Fiber Intake! (Without Difficulty Or Discomfort)
- That said, if you’re looking for things to cut, sugar is a top candidate (and red meat is in clear second place albeit some way below)
When it comes to exercise, get your 10,000 daily steps in (actually, science says 8,000 steps is fine), and consider adding HIIT per our above article, when you feel like adding that in. As for that about the steps:
When it comes to sleep, if you’re taking care of the above things, and set a regular early wake-up time that you do not deviate from, then this will probably take care of itself, if you don’t have a sleep-inconvenient lifestyle (e.g. shift work, just had a baby, etc) or a sleep disorder.
For further pointers, see: 10 Tips for Better Sleep: Starting In The Morning
Take care!
Don’t Forget…
Did you arrive here from our newsletter? Don’t forget to return to the email to continue learning!
Learn to Age Gracefully
Join the 98k+ American women taking control of their health & aging with our 100% free (and fun!) daily emails:
-
Pomegranate vs Cranberries – Which is Healthier?
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
Our Verdict
When comparing pomegranate to cranberries, we picked the pomegranate.
Why?
Starting with the macros: pomegranate has nearly 4x the protein (actually quite a lot for a fruit, but this is not too surprising—it’s because we are eating the seeds!), and slightly more carbs and fiber. Their glycemic indices are comparable, both being low GI foods. While both of these fruits have excellent macro profiles, we say the pomegranate is slightly better, because of the protein, and when it comes to the carbs and fiber, since they balance each other out, we’ll go with the option that’s more nutritionally dense. We like foods that add more nutrients!
In the category of vitamins, pomegranate is higher in vitamins B1, B2, B3, B5, B6, B9, K, and choline, while cranberry is higher in vitamins A, C, and E. Both are very respectable profiles, but pomegranate wins on strength of numbers (and also some higher margins of difference).
When it comes to minerals, it is not close; pomegranate is higher in calcium, copper, iron, magnesium, phosphorus, potassium, selenium, and zinc, while cranberry is higher in manganese. An easy win for pomegranate here.
Both of these fruits have additional “special” properties, though it’s worth noting that:
- pomegranate’s bonus properties, which are too many to list here, but we link to an article below, are mostly in its peel (so dry it, and grind it into a powder supplement, that can be worked into foods, or used like an instant fruit tea, just without the sugar)
- cranberries’ bonus properties (including: famously very good at reducing UTI risk) come with some warnings, including that they may increase the risk of kidney stones if you are prone to such, and also that cranberries have anti-clotting effects, which are great for heart health but can be a risk of you’re on blood thinners or have a bleeding disorder.
You can read about both of these fruits’ special properties in more detail below:
Want to learn more?
You might like to read:
- Health Benefits Of Cranberries (But: You’d Better Watch Out)
- Pomegranate’s Health Gifts Are Mostly In Its Peel
Take care!
Don’t Forget…
Did you arrive here from our newsletter? Don’t forget to return to the email to continue learning!
Learn to Age Gracefully
Join the 98k+ American women taking control of their health & aging with our 100% free (and fun!) daily emails:
-
Walk Yourself Happy – by Dr. Julia Bradbury
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
Notwithstanding her (honorary) doctorate, Dr. Bradbury is not, in fact, a scientist. But…
- She has a lot of experience walking all around the world, and her walking habit has seen her through all manner of things, from stress and anxiety to cancer and grief and more.
- She does, throughout this book, consult many scientists and other experts (indeed, some we’ve featured here before at 10almonds), so we still get quite a dose of science too.
The writing style of this book is… Compelling. Honestly, the biggest initial barrier to you getting out of the door will be putting this book down first.If you have good self-discipline, you might make it last longer by treating yourself to a chapter per day
Bottom line: you probably don’t need this book to know how to go for a walk, but it will motivate, inspire, and even inform you of how to get the most out of it. Treat yourself!
Click here to check out Walk Yourself Happy, and prepare for a new healthy habit!
Don’t Forget…
Did you arrive here from our newsletter? Don’t forget to return to the email to continue learning!
Learn to Age Gracefully
Join the 98k+ American women taking control of their health & aging with our 100% free (and fun!) daily emails: