Behaving During the Holidays
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
It’s Q&A Day at 10almonds!
Have a question or a request? You can always hit “reply” to any of our emails, or use the feedback widget at the bottom!
In cases where we’ve already covered something, we might link to what we wrote before, but will always be happy to revisit any of our topics again in the future too—there’s always more to say!
As ever: if the question/request can be answered briefly, we’ll do it here in our Q&A Thursday edition. If not, we’ll make a main feature of it shortly afterwards!
So, no question/request too big or small
❝It’s hard to “behave” when it comes to holiday indulging…I’m on a low sodium, sugar restricted regimen from my doctor. Trying to get interested in bell peppers as a snack…wish me luck!❞
Good luck! Other low sodium, low sugar snacks include:
- Nuts! Unsalted, of course. We’re biased towards almonds 😉
- Mixed nuts are an especially healthy way to snack, though (assuming you don’t have an allergy, obviously)
- Air-popped popcorn (you can season it, just not with salt/sugar!)
- Fruit (but not fruit juice; it has to be in solid form)
- Peas (not a classic snack food, we know, but they can be enjoyed many ways)
- Seriously, try them frozen or raw! Frozen/raw peas are a great sweet snack.
- Chickpeas are great dried/roasted, by the way, and give much of the same pleasure as a salty snack without being salty! Obviously, this means cooking them without salt, but that’s fine, or if using tinned, choose “in water” rather than “in brine”
- Hummus is also a great healthy snack (check the ingredients for salt if not making it yourself, though) and can be enjoyed as a dip using raw vegetables (celery, carrot sticks, cruciferous vegetables, whatever you prefer)
Enjoy!
Don’t Forget…
Did you arrive here from our newsletter? Don’t forget to return to the email to continue learning!
Recommended
Learn to Age Gracefully
Join the 98k+ American women taking control of their health & aging with our 100% free (and fun!) daily emails:
-
Get On It! – by Jane Aronovitch, Miriane Taylor, & Colleen Craig
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
Balance is important; without it, we die early. That’s quite a strong selling point for improving one’s balance, but why this book in particular?
This is—with one drawback—the best book of balance ball exercises we’ve seen. Notwithstanding the cover photo, many exercises do, by the way, involve standing on it with one or both feet, doing various kinds of squats, lunges, get-ups, and so forth. The ball (it’s not really a ball so much as an oblate hemisphere) can also be flipped and used the other way around, with a flat platform that will now wobble per your weight distribution, and train balance in different ways (dome-up trains large stabilizing muscles more; platform-up trains smaller stabilizing muscles more).
Indeed, that’s where the brand name Bosu, often stylized “BOSU”, comes from: both sides up!
So, what’s the drawback? Alas, the photos are black and white, which means in some cases they’re not as clear as they could be. Nothing that will prevent understanding the exercises, which are well-explained in any case, but it does mean that sometimes it’s necessary to look closely to see which leg is in front of the other for a given exercise, for example.
Still, with 80 different exercises it really does cover the whole body, and even gives workout program varieties for those who want that, including targeted to particular areas, e.g. lower body, core, upper body, or complete.
Bottom line: if you’d like to improve your balance (and have, or are willing to acquire, a balance ball like the Bosu), then this book will give you everything else you need in that regard.
Share This Post
-
What’s the difference between autism and Asperger’s disorder?
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
Swedish climate activist Greta Thunberg describes herself as having Asperger’s while others on the autism spectrum, such as Australian comedian Hannah Gatsby, describe themselves as “autistic”. But what’s the difference?
Today, the previous diagnoses of “Asperger’s disorder” and “autistic disorder” both fall within the diagnosis of autism spectrum disorder, or ASD.
Autism describes a “neurotype” – a person’s thinking and information-processing style. Autism is one of the forms of diversity in human thinking, which comes with strengths and challenges.
When these challenges become overwhelming and impact how a person learns, plays, works or socialises, a diagnosis of autism spectrum disorder is made.
Where do the definitions come from?
The Diagnostic and Statistical Manual of Mental Disorders (DSM) outlines the criteria clinicians use to diagnose mental illnesses and behavioural disorders.
Between 1994 and 2013, autistic disorder and Asperger’s disorder were the two primary diagnoses related to autism in the fourth edition of the manual, the DSM-4.
In 2013, the DSM-5 collapsed both diagnoses into one autism spectrum disorder.
How did we used to think about autism?
The two thinkers behind the DSM-4 diagnostic categories were Baltimore psychiatrist Leo Kanner and Viennese paediatrician Hans Asperger. They described the challenges faced by people who were later diagnosed with autistic disorder and Asperger’s disorder.
Kanner and Asperger observed patterns of behaviour that differed to typical thinkers in the domains of communication, social interaction and flexibility of behaviour and thinking. The variance was associated with challenges in adaptation and distress.
Kanner and Asperger described different thinking patterns in children with autism.
Roman Nerud/ShutterstockBetween the 1940s and 1994, the majority of those diagnosed with autism also had an intellectual disability. Clinicians became focused on the accompanying intellectual disability as a necessary part of autism.
The introduction of Asperger’s disorder shifted this focus and acknowledged the diversity in autism. In the DSM-4 it superficially looked like autistic disorder and Asperger’s disorder were different things, with the Asperger’s criteria stating there could be no intellectual disability or delay in the development of speech.
Today, as a legacy of the recognition of the autism itself, the majority of people diagnosed with autism spectrum disorder – the new term from the DSM-5 – don’t a have an accompanying intellectual disability.
What changed with ‘autism spectrum disorder’?
The move to autism spectrum disorder brought the previously diagnosed autistic disorder and Asperger’s disorder under the one new diagnostic umbrella term.
It made clear that other diagnostic groups – such as intellectual disability – can co-exist with autism, but are separate things.
The other major change was acknowledging communication and social skills are intimately linked and not separable. Rather than separating “impaired communication” and “impaired social skills”, the diagnostic criteria changed to “impaired social communication”.
The introduction of the spectrum in the diagnostic term further clarified that people have varied capabilities in the flexibility of their thinking, behaviour and social communication – and this can change in response to the context the person is in.
Why do some people prefer the old terminology?
Some people feel the clinical label of Asperger’s allowed a much more refined understanding of autism. This included recognising the achievements and great societal contributions of people with known or presumed autism.
The contraction “Aspie” played an enormous part in the shift to positive identity formation. In the time up to the release of the DSM-5, Tony Attwood and Carol Gray, two well known thinkers in the area of autism, highlighted the strengths associated with “being Aspie” as something to be proud of. But they also raised awareness of the challenges.
What about identity-based language?
A more recent shift in language has been the reclamation of what was once viewed as a slur – “autistic”. This was a shift from person-first language to identity-based language, from “person with autism spectrum disorder” to “autistic”.
The neurodiversity rights movement describes its aim to push back against a breach of human rights resulting from the wish to cure, or fundamentally change, people with autism.
Autism is one of the forms of diversity in human thinking, which comes with strengths and challenges.
Alex and Maria photo/ShutterstockThe movement uses a “social model of disability”. This views disability as arising from societies’ response to individuals and the failure to adjust to enable full participation. The inherent challenges in autism are seen as only a problem if not accommodated through reasonable adjustments.
However the social model contrasts itself against a very outdated medical or clinical model.
Current clinical thinking and practice focuses on targeted supports to reduce distress, promote thriving and enable optimum individual participation in school, work, community and social activities. It doesn’t aim to cure or fundamentally change people with autism.
A diagnosis of autism spectrum disorder signals there are challenges beyond what will be solved by adjustments alone; individual supports are also needed. So it’s important to combine the best of the social model and contemporary clinical model.
Andrew Cashin, Professor of Nursing, School of Health and Human Sciences, Southern Cross University
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Share This Post
-
Mastering Gut Health for Women – by Karín Feltman
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
The author, a registered nurse, has a focus on holistic health, and in this book it’s all about wellness from the inside out.
To effect this, she lays out a 12-week program of transformations:
- Week 1: transform your knowledge
- Week 2: transform your brain
- Week 3: transform your digestion
- Week 4: transform your immunity
- Week 5: transform your emotions
- Week 6: transform your sleep
- Week 7: transform your energy/vitality
- Week 8: transform your activity
- Week 9: transform your hormones
- Week 10: transform your diet
- Week 11: transform your weight
- Week 12: transform your habits
Which all adds up to quite a comprehensive overall transformation!
Of course, it’s possible you might want to implement everything at once; an exciting prospect for sure, but oftentimes it really is best to just change one thing at once before moving on; that way it’s a lot more likely to stick, and that’s why she presents it in this format.
On the other hand, maybe you might want to take longer than the 12 weeks, if for example it takes you more than a week to do a certain part. That’s fine too, though for most people without serious constraints (or suffering some unexpected major interruption to your usual life), the 12-week program should be quite doable as-is.
The style is personable and friendly, albeit with frequent references to science and appropriate citations.
Bottom line: the title centers gut health, and so does the book itself, but this is truly a holistic approach that goes far beyond the gut, which makes it even more worthwhile.
Click here to check out Mastering Gut Health For Women, and master gut health for yourself!
Share This Post
Related Posts
-
I have a stuffy nose, how can I tell if it’s hay fever, COVID or something else?
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
Hay fever (also called allergic rhinitis) affects 24% of Australians. Symptoms include sneezing, a runny nose (which may feel blocked or stuffy) and itchy eyes. People can also experience an itchy nose, throat or ears.
But COVID is still spreading, and other viruses can cause cold-like symptoms. So how do you know which one you’ve got?
Lysenko Andrii/Shutterstock Remind me, how does hay fever cause symptoms?
Hay fever happens when a person has become “sensitised” to an allergen trigger. This means a person’s body is always primed to react to this trigger.
Triggers can include allergens in the air (such as pollen from trees, grasses and flowers), mould spores, animals or house dust mites which mostly live in people’s mattresses and bedding, and feed on shed skin.
When the body is exposed to the trigger, it produces IgE (immunoglobulin E) antibodies. These cause the release of many of the body’s own chemicals, including histamine, which result in hay fever symptoms.
People who have asthma may find their asthma symptoms (cough, wheeze, tight chest or trouble breathing) worsen when exposed to airborne allergens. Spring and sometimes into summer can be the worst time for people with grass, tree or flower allergies.
However, animal and house dust mite symptoms usually happen year-round.
Ryegrass pollen is a common culprit. bangku ceria/Shutterstock What else might be causing my symptoms?
Hay fever does not cause a fever, sore throat, muscle aches and pains, weakness, loss of taste or smell, nor does it cause you to cough up mucus.
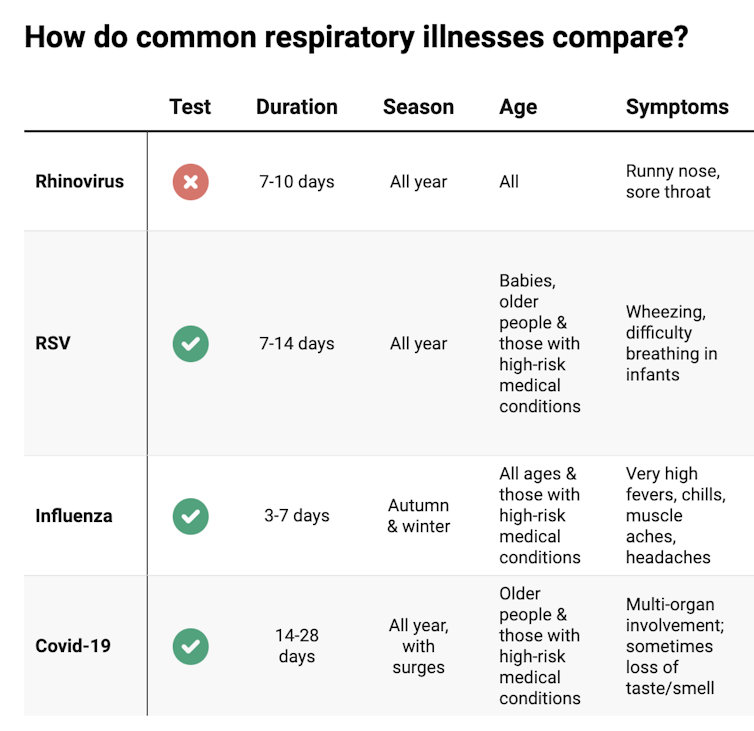
These symptoms are likely to be caused by a virus, such as COVID, influenza, respiratory syncytial virus (RSV) or a “cold” (often caused by rhinoviruses). These conditions can occur all year round, with some overlap of symptoms:
Natasha Yates/The Conversation COVID still surrounds us. RSV and influenza rates appear higher than before the COVID pandemic, but it may be due to more testing.
So if you have a fever, sore throat, muscle aches/pains, weakness, fatigue, or are coughing up mucus, stay home and avoid mixing with others to limit transmission.
People with COVID symptoms can take a rapid antigen test (RAT), ideally when symptoms start, then isolate until symptoms disappear. One negative RAT alone can’t rule out COVID if symptoms are still present, so test again 24–48 hours after your initial test if symptoms persist.
You can now test yourself for COVID, RSV and influenza in a combined RAT. But again, a negative test doesn’t rule out the virus. If your symptoms continue, test again 24–48 hours after the previous test.
If it’s hay fever, how do I treat it?
Treatment involves blocking the body’s histamine release, by taking antihistamine medication which helps reduce the symptoms.
Doctors, nurse practitioners and pharmacists can develop a hay fever care plan. This may include using a nasal spray containing a topical corticosteroid to help reduce the swelling inside the nose, which causes stuffiness or blockage.
Nasal sprays need to delivered using correct technique and used over several weeks to work properly. Often these sprays can also help lessen the itchy eyes of hay fever.
Drying bed linen and pyjamas inside during spring can lessen symptoms, as can putting a smear of Vaseline in the nostrils when going outside. Pollen sticks to the Vaseline, and gently blowing your nose later removes it.
People with asthma should also have an asthma plan, created by their doctor or nurse practitioner, explaining how to adjust their asthma reliever and preventer medications in hay fever seasons or on allergen exposure.
People with asthma also need to be alert for thunderstorms, where pollens can burst into tinier particles, be inhaled deeper in the lungs and cause a severe asthma attack, and even death.
What if it’s COVID, RSV or the flu?
Australians aged 70 and over and others with underlying health conditions who test positive for COVID are eligible for antivirals to reduce their chance of severe illness.
Most other people with COVID, RSV and influenza will recover at home with rest, fluids and paracetamol to relieve symptoms. However some groups are at greater risk of serious illness and may require additional treatment or hospitalisation.
For RSV, this includes premature infants, babies 12 months and younger, children under two who have other medical conditions, adults over 75, people with heart and lung conditions, or health conditions that lessens the immune system response.
For influenza, people at higher risk of severe illness are pregnant women, Aboriginal people, people under five or over 65 years, or people with long-term medical conditions, such as kidney, heart, lung or liver disease, diabetes and decreased immunity.
If you’re concerned about severe symptoms of COVID, RSV or influenza, consult your doctor or call 000 in an emergency.
If your symptoms are mild but persist, and you’re not sure what’s causing them, book an appointment with your doctor or nurse practitioner. Although hay fever season is here, we need to avoid spreading other serious infectious.
For more information, you can call the healthdirect helpline on 1800 022 222 (known as NURSE-ON-CALL in Victoria); use the online Symptom Checker; or visit healthdirect.gov.au or the Australian Society of Clinical Immunology and Allergy.
Deryn Thompson, Eczema and Allergy Nurse; Lecturer, University of South Australia
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Don’t Forget…
Did you arrive here from our newsletter? Don’t forget to return to the email to continue learning!
Learn to Age Gracefully
Join the 98k+ American women taking control of their health & aging with our 100% free (and fun!) daily emails:
-
Codependency Isn’t What Most People Think It Is
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
Codependency isn’t what most people think it is
In popular parlance, people are often described as “codependent” when they rely on each other to function normally. That’s interdependent mutualism, and while it too can become a problem if a person is deprived of their “other half” and has no idea how to do laundry and does not remember to take their meds, it’s not codependency.
Codependency finds its origins in the treatment and management of alcoholism, and has been expanded to encompass other forms of relationships with dependence on substances and/or self-destructive behaviors—which can be many things, including the non-physical, for example a pattern of irresponsible impulse-spending, or sabotaging one’s own relationship(s).
We’ll use the simplest example, though:
- Person A is (for example) an alcoholic. They have a dependency.
- Person B, married to A, is not an alcoholic. However, their spouse’s dependency affects them greatly, and they do what they can to manage that, and experience tension between wanting to “save” their spouse, and wanting their spouse to be ok, which latter, superficially, often means them having their alcohol.
Person B is thus said to be “codependent”.
The problem with codependency
The problems of codependency are mainly twofold:
- The dependent partner’s dependency is enabled and thus perpetuated by the codependent partner—they might actually have to address their dependency, if it weren’t for their partner keeping them from too great a harm (be it financially, socially, psychologically, medically, whatever)
- The codependent partner is not having a good time of it either. They have the stress of two lives with the resources (e.g. time) of one. They are stressing about something they cannot control, understandably worrying about their loved one, and, worse: every action they might take to “save” their loved one by reducing the substance use, is an action that makes their partner unhappy, and causes conflict too.
Note: codependency is often a thing in romantic relationships, but it can appear in other relationships too, e.g. parent-child, or even between friends.
See also: Development and validation of a revised measure of codependency
How to deal with this
If you find yourself in a codependent position, or are advising someone who is, there are some key things that can help:
- Be a nurturer, not a rescuer. It is natural to want to “rescue” someone we care about, but there are some things we cannot do for them. Instead, we must look for ways to build their strength so that they can take the steps that only they can take to fix the problem.
- Establish boundaries. Practise saying “no”, and also be clear over what things you can and cannot control—and let go of the latter. Communicate this, though. An “I’m not the boss of you” angle can prompt a lot of people to take more personal responsibility.
- Schedule time for yourself. You might take some ideas from our previous tangentially-related article:
How To Avoid Carer Burnout (Without Dropping Care)
Want to read more?
That’s all we have space for today, but here’s a very useful page with a lot of great resources (including questionnaires and checklist and things, in case you’re thinking “is it, or…?”)
Don’t Forget…
Did you arrive here from our newsletter? Don’t forget to return to the email to continue learning!
Learn to Age Gracefully
Join the 98k+ American women taking control of their health & aging with our 100% free (and fun!) daily emails:
-
‘It’s okay to poo at work’: new health campaign highlights a common source of anxiety
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
For most people, the daily or near-daily ritual of having a bowel motion is not something we give a great deal of thought to. But for some people, the need to do a “number two” in a public toilet or at work can be beset with significant stress and anxiety.
In recognition of the discomfort people may feel around passing a bowel motion at work, the Queensland Department of Health recently launched a social media campaign with the message “It’s okay to poo at work”.
The campaign has gained significant traction on Instagram and Facebook. It has been praised by health and marketing experts for its humorous handling of a taboo topic.
A colourful Instagram post is accompanied by a caption warning of the health risks of “holding it in”, including haemorrhoids and other gastrointestinal problems. The caption also notes:
If you find it extremely difficult to poo around other people, you might have parcopresis.
Queensland Health/Instagram What is parcopresis?
Parcopresis, sometimes called “shy bowel”, occurs when people experience a difficulty or inability to poo in public toilets due to fear of perceived scrutiny by others.
People with parcopresis may find it difficult to go to the toilet in public places such as shopping centres, restaurants, at work or at school, or even at home when friends or family are around.
They may fear being judged by others about unpleasant smells or sounds when they have a bowel motion, or how long they take to go, for example.
Living with a gastrointestinal condition (at least four in ten Australians do) may contribute to parcopresis due to anxiety about the need to use a toilet frequently, and perceived judgment from others when doing so. Other factors, such as past negative experiences or accessibility challenges, may also play a role.
Some people may feel uncomfortable about using the toilet at work. Motortion Films/Shutterstock For sufferers, anxiety can present in the form of a faster heart rate, rapid breathing, sweating, muscle tension, blushing, nausea, trembling, or a combination of these symptoms. They may experience ongoing worry about situations where they may need to use a public toilet.
Living with parcopresis can affect multiple domains of life and quality of life overall. For example, sufferers may have difficulties relating to employment, relationships and social life. They might avoid travelling or attending certain events because of their symptoms.
How common is parcopresis?
We don’t really know how common parcopresis is, partly due to the difficulty of evaluating this behaviour. It’s not necessarily easy or appropriate to follow people around to track whether they use or avoid public toilets (and their reasons if they do). Also, observing individual bathroom activities may alter the person’s behaviour.
I conducted a study to try to better understand how common parcopresis is. The study involved 714 university students. I asked participants to respond to a series of vignettes, or scenarios.
In each vignette participants were advised they were at a local shopping centre and they needed to have a bowel motion. In the vignettes, the bathrooms (which had been recently cleaned) had configurations of either two or three toilet stalls. Each vignette differed by the configuration of stalls available.
The rate of avoidance was just over 14% overall. But participants were more likely to avoid using the toilet when the other stalls were occupied.
Around 10% avoided going when all toilets were available. This rose to around 25% when only the middle of three toilets was available. Men were significantly less likely to avoid going than women across all vignettes.
For those who avoided the toilet, many either said they would go home to poo, use an available disabled toilet, or come back when the bathroom was empty.
Parcopresis at work
In occupational settings, the rates of anxiety about using shared bathrooms may well be higher for a few reasons.
For example, people may feel more self-conscious about their bodily functions being heard or noticed by colleagues, compared to strangers in a public toilet.
People may also experience guilt, shame and fear about being judged by colleagues or supervisors if they need to make extended or frequent visits to the bathroom. This may particularly apply to people with a gastrointestinal condition.
Reducing restroom anxiety
Using a public toilet can understandably cause some anxiety or be unpleasant. But for a small minority of people it can be a real problem, causing severe distress and affecting their ability to engage in activities of daily living.
If doing a poo in a toilet at work or another public setting causes you anxiety, be kind to yourself. A number of strategies might help:
- identify and challenge negative thoughts about using public toilets and remind yourself that using the bathroom is normal, and that most people are not paying attention to others in the toilets
- try to manage stress through relaxation techniques such as deep breathing and progressive muscle relaxation, which involves tensing and relaxing different muscles around the body
- engaging in gradual exposure can be helpful, which means visiting public toilets at different times and locations, so you can develop greater confidence in using them
- use grounding or distraction techniques while going to the toilet. These might include listening to music, watching something on your phone, or focusing on your breathing.
If you feel parcopresis is having a significant impact on your life, talk to your GP or a psychologist who can help identify appropriate approaches to treatment. This might include cognitive behavioural therapy.
Simon Robert Knowles, Associate Professor and Clinical Psychologist, Swinburne University of Technology
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Don’t Forget…
Did you arrive here from our newsletter? Don’t forget to return to the email to continue learning!
Learn to Age Gracefully
Join the 98k+ American women taking control of their health & aging with our 100% free (and fun!) daily emails: