Food and exercise can treat depression as well as a psychologist, our study found. And it’s cheaper
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
Around 3.2 million Australians live with depression.
At the same time, few Australians meet recommended dietary or physical activity guidelines. What has one got to do with the other?
Our world-first trial, published this week, shows improving diet and doing more physical activity can be as effective as therapy with a psychologist for treating low-grade depression.
Previous studies (including our own) have found “lifestyle” therapies are effective for depression. But they have never been directly compared with psychological therapies – until now.
Amid a nation-wide shortage of mental health professionals, our research points to a potential solution. As we found lifestyle counselling was as effective as psychological therapy, our findings suggest dietitians and exercise physiologists may one day play a role in managing depression.

What did our study measure?
During the prolonged COVID lockdowns, Victorians’ distress levels were high and widespread. Face-to-face mental health services were limited.
Our trial targeted people living in Victoria with elevated distress, meaning at least mild depression but not necessarily a diagnosed mental disorder. Typical symptoms included feeling down, hopeless, irritable or tearful.
We partnered with our local mental health service to recruit 182 adults and provided group-based sessions on Zoom. All participants took part in up to six sessions over eight weeks, facilitated by health professionals.
Half were randomly assigned to participate in a program co-facilitated by an accredited practising dietitian and an exercise physiologist. That group – called the lifestyle program – developed nutrition and movement goals:

- eating a wide variety of foods
- choosing high-fibre plant foods
- including high quality fats
- limiting discretionary foods, such as those high in saturated fats and added sugars
- doing enjoyable physical activity.
The second group took part in psychotherapy sessions convened by two psychologists. The psychotherapy program used cognitive behavioural therapy (CBT), the gold standard for treating depression in groups and when delivered remotely.
In both groups, participants could continue existing treatments (such as taking antidepressant medication). We gave both groups workbooks and hampers. The lifestyle group received a food hamper, while the psychotherapy group received items such as a colouring book, stress ball and head massager.
Lifestyle therapies just as effective
We found similar results in each program.
At the trial’s beginning we gave each participant a score based on their self-reported mental health. We measured them again at the end of the program.
Over eight weeks, those scores showed symptoms of depression reduced for participants in the lifestyle program (42%) and the psychotherapy program (37%). That difference was not statistically or clinically meaningful so we could conclude both treatments were as good as each other.
There were some differences between groups. People in the lifestyle program improved their diet, while those in the psychotherapy program felt they had increased their social support – meaning how connected they felt to other people – compared to at the start of the treatment.
Participants in both programs increased their physical activity. While this was expected for those in the lifestyle program, it was less expected for those in the psychotherapy program. It may be because they knew they were enrolled in a research study about lifestyle and subconsciously changed their activity patterns, or it could be a positive by-product of doing psychotherapy.

There was also not much difference in cost. The lifestyle program was slightly cheaper to deliver: A$482 per participant, versus $503 for psychotherapy. That’s because hourly rates differ between dietitians and exercise physiologists, and psychologists.
What does this mean for mental health workforce shortages?
Demand for mental health services is increasing in Australia, while at the same time the workforce faces worsening nation-wide shortages.
Psychologists, who provide about half of all mental health services, can have long wait times. Our results suggest that, with the appropriate training and guidelines, allied health professionals who specialise in diet and exercise could help address this gap.
Lifestyle therapies can be combined with psychology sessions for multi-disciplinary care. But diet and exercise therapies could prove particularly effective for those on waitlists to see a psychologists, who may be receiving no other professional support while they wait.
Many dietitians and exercise physiologists already have advanced skills and expertise in motivating behaviour change. Most accredited practising dietitians are trained in managing eating disorders or gastrointestinal conditions, which commonly overlap with depression.
There is also a cost argument. It is overall cheaper to train a dietitian ($153,039) than a psychologist ($189,063) – and it takes less time.
Potential barriers
Australians with chronic conditions (such as diabetes) can access subsidised dietitian and exercise physiologist appointments under various Medicare treatment plans. Those with eating disorders can also access subsidised dietitian appointments. But mental health care plans for people with depression do not support subsidised sessions with dietitians or exercise physiologists, despite peak bodies urging them to do so.
Increased training, upskilling and Medicare subsidies would be needed to support dietitians and exercise physiologists to be involved in treating mental health issues.
Our training and clinical guidelines are intended to help clinicians practising lifestyle-based mental health care within their scope of practice (activities a health care provider can undertake).
Future directions
Our trial took place during COVID lockdowns and examined people with at least mild symptoms of depression who did not necessarily have a mental disorder. We are seeking to replicate these findings and are now running a study open to Australians with mental health conditions such as major depression or bipolar disorder.
If this article has raised issues for you, or if you’re concerned about someone you know, call Lifeline on 13 11 14.
Adrienne O’Neil, Professor, Food & Mood Centre, Deakin University and Sophie Mahoney, Associate Research Fellow, Food and Mood Centre, Deakin University
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Don’t Forget…
Did you arrive here from our newsletter? Don’t forget to return to the email to continue learning!
Recommended
Learn to Age Gracefully
Join the 98k+ American women taking control of their health & aging with our 100% free (and fun!) daily emails:
-
What are house dust mites and how do I know if I’m allergic to them?
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
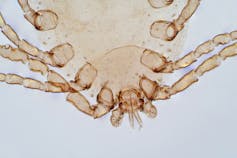
People often believe they are allergic to house dust. But of the 20% of Australians suffereing with allergies, a number are are actually allergic to microscopic house dust mites.
House dust mites belong to the same family as spiders and ticks. They measure just 0.2-0.3 mm, with 50 fitting on a single pinhead. They live for 65–100 days, and females lay 60–100 eggs in their life.
Some 50 house dust mites can fit on one pinhead. Choksawatdikorn/Shutterstock House dust mites love temperate climates and humidity. They feed off the skin cells we and animals shed, as well as mould, which they digest using special enzymes. These enzymes are excreted in their poo about 20 times a day. They also shed fragments of their exoskeletons.
All these fragments trigger allergies in people with this type of allergic rhinitis (which is also known as hay fever)
shuttertock. PeopleImages.com – Yuri A/Shutterstock What are the symptoms?
When people with house dust mite allergy inhale the allergens, they penetrate the mucous membranes of the airways and eyes. Their body recognises the allergens as a threat, releasing chemicals including one called histamine.
This causes symptoms including a runny nose, an itchy nose, eyes and throat, sneezing, coughing and a feeling of mucus at the back of your throat (known as a post-nasal drip).
People with this type of allergy usually mouth breath, snore, rub their nose constantly (creating a nasal crease called the “dust mite salute”) and have dark shadows under their eyes.
House dust mite allergy can also cause poor sleep, constant tiredness, reduced concentration at work or school and lower quality of life.
For people with eczema, their damaged skin barrier can allow house dust mite proteins in. This prompts immune cells in the skin to release chemicals which make already flared skin become redder, sorer and itchier, especially in children.
Symptoms of house dust mite allergy occur year round, and are often worse after going to bed and when waking in the morning. But people with house dust mite allergy and pollen allergies find their year-round symptoms worsen in spring.
How is it diagnosed?
House dust mite allergy symptoms often build up over months, or even years before people seek help. But an accurate diagnosis means you can not only access the right treatment – it’s also vital for minimising exposure.
Your clinician can talk you through treatment options and how to minimise exposure. Monkey Business Images/Shutterstock Doctor and nurse practitioners can order a blood test to check for house dust mite allergy.
Alternatively, health care providers with specialised allergy training can perform skin prick tests. This involves placing drops of the allergens on the arm, along with a positive and negative “control”. After 15 minutes, those who test positive will have developed a mosquito bite-like mark.
How is it treated?
Medication options include one or a combination of:
- daily non-sedating antihistamines
- a steroid nasal spray
- allergy eye drops.
Your health care professional will work with you to develop a rhinitis (hay fever) medical management plan to reduce your symptoms. If you’re using a nasal spray, your health provider will show you how to use it, as people often use it incorrectly.
If you also have asthma or eczema which is worsened by dust mites, your health provider will adapt your asthma action plan or eczema care plan accordingly.
If you experience severe symptoms, a longer-term option is immunotherapy. This aims to gradually turn off your immune system’s ability to recognise house dust mites as a harmful allergen.
Immunotherapy involves taking either a daily sublingual tablet, under the tongue, or a series of injections. Injections require monthly attendances over three years, after the initial weekly build-up phase.
These are effective, but are costly (as well as time-consuming). So it’s important to weigh up the potential benefits and downsides with your health-care provider.
How can you minimise house dust mites?
There are also important allergy minimisation measures you can take to reduce allergens in your home.
Each week, wash your bedding and pyjamas in hot water (over 60°C). This removes house dust mite eggs and debris.
Opt for doonas, covers or quilts that can be washed in hot water above 60°C. Alternatively, low-cost waterproof or leak proof covers can keep house dust mites out.
If you can, favour blinds and wood floors over curtains and carpet. Dust blinds and surfaces with a damp cloth each week and vacuum while wearing a mask, or have someone else do it, as house dust mites can become airborne during cleaning.
But beware of costly products with big marketing budgets and little evidence to support their use. A new mattress, for example, will always be house dust mite-free. But once slept on, the house dust mite life cycle can start.
Mattress protectors and toppers commonly claim to be “hypoallergenic”, “anti-allergy” or “allergy free”. But their pore sizes are not small enough to keep house dust mites and their poo out, or shed skin going through.
Sprays claiming to kill mites require so much spray to penetrate the product that it’s likely to become wet, may smell like the spray and, unless dried properly, may grow mould.
Finally, claims that expensive vacuum cleaners can extract all the house dust mites are unsubstantiated.
For more information, visit healthdirect.gov.au or the Australian Society of Clinical Immunology and Allergy.
Deryn Lee Thompson, Eczema and Allergy Nurse; Lecturer, University of South Australia
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Share This Post
-
Improve Your Insulin Sensitivity!
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
We’ve written before about blood sugar management, for example:
10 Ways To Balance Blood Sugars ← this one really is the most solid foundation possible; if you do nothing else, do these 10 things!
And as for why we care:
Good (Or Bad) Health Starts With Your Blood
…because the same things that cause type 2 diabetes, go on to cause many other woes, with particularly strong comorbidities in the case of Alzheimer’s disease and other dementias, as well as heart disease of various kinds, and a long long laundry list of immune dysfunctions / inflammatory disorders in general.
In short, if you can’t keep your blood sugars even, the rest of your health will fall like so many dominoes.
Getting a baseline
Are you counting steps? Counting calories? Monitoring your sleep? Heart rate zones? These all have their merits:
- Steps: One More Resource Against Osteoporosis!
- Calories: Is Cutting Calories The Key To Healthy Long Life?
- Sleep: A Head-To-Head Of Google and Apple’s Top Apps For Getting Your Head Down
- Heart Rate Zones: Heart Rate Zones, Oxalates, & More
But something far fewer people do unless they have diabetes or are very enthusiastic about personal health, is to track blood sugars:
Here’s how: Track Your Blood Sugars For Better Personalized Health
And for understanding some things to watch out for when using a continuous glucose monitor:
Continuous Glucose Monitors Without Diabetes: Pros & Cons
Writer’s anecdote: I decided to give one a try for a few months, and so far it has been informative, albeit unexciting. It seems that with my diet (mostly whole-foods plant based, though I do have a wholegrain wheat product about twice per week (usually: flatbread once, pasta once) which is… Well, we could argue it’s whole-food plant based, but let’s be honest, it’s a little processed), my blood sugars don’t really have spikes at all; the graph looks more like gently rolling low hills (which is good). However! Even so, by experimenting with it, I can see for myself what differences different foods/interventions make to my blood sugars, which is helpful, and it also improves my motivation for intermittent fasting. It also means that if I think “hmm, my energy levels are feeling low; I need a snack” I can touch my phone to my arm and find out if that is really the reason (so far, it hasn’t been). I expect that as I monitor my blood sugars continuously and look at the data frequently, I’ll start to get a much more intuitive feel for my own blood sugars, in much the same way I can generally intuit my hormone levels correctly after years of taking-and-testing.
So much for blood sugars. Now, what about insulin?
Step Zero
If taking care of blood sugars is step one, then taking care of insulin is step zero.
Often’s it’s viewed the other way around: we try to keep our blood sugars balanced, to reduce the need for our bodies to produce so much insulin that it gets worn out. And that’s good and fine, but…
To quote what we wrote when reviewing “Why We Get Sick” last month:
❝Dr. Bikman makes the case that while indeed hyper- or hypoglycemia bring their problems, mostly these are symptoms rather than causes, and the real culprit is insulin resistance, and this is important for two main reasons:
- Insulin resistance occurs well before the other symptoms set in (which means: it is the thing that truly needs to be nipped in the bud; if your fasting blood sugars are rising, then you missed “nipping it in the bud” likely by a decade or more)
- Insulin resistance causes more problems than “mere” hyperglycemia (the most commonly-known result of insulin resistance) does, so again, it really needs to be considered separately from blood sugar management.
This latter, Dr. Bikman goes into in great detail, linking insulin resistance (even if blood sugar levels are normal) to all manner of diseases (hence the title).
You may be wondering: how can blood sugar levels be normal, if we have insulin resistance?
And the answer is that for as long as it is still able, your pancreas will just faithfully crank out more and more insulin to deal with the blood sugar levels that would otherwise be steadily rising. Since people measure blood sugar levels much more regularly than anyone checks for actual insulin levels, this means that one can be insulin resistant for years without knowing it, until finally the pancreas is no longer able to keep up with the demand—then that’s when people finally notice.❞
You can read the full book review here:
Now, testing for insulin is not so quick, easy, or accessible as testing for glucose, but it can be worthwhile to order such a test—because, as discussed, your insulin levels could be high even while your blood sugars are still normal, and it won’t be until the pancreas finally reaches breaking point that your blood sugars show it.
So, knowing your insulin levels can help you intervene before your pancreas reaches that breaking point.
We can’t advise on local services available for ordering blood tests (because they will vary depending on location), but a simple Google search should suffice to show what’s available in your region.
Once you know your insulin levels (or even if you don’t, but simply take the principled position that improving insulin sensitivity will be good regardless), you can set about managing them.
Insulin sensitivity is important, because the better it is (higher insulin sensitivity), the less insulin the pancreas has to make to tidy up the same amount of glucose into places that are good for it to go—which is good. In contrast, the worse it is (higher insulin resistance), the more insulin the pancreas has to make to do the same blood sugar management. Which is bad.
What to do about it
We imagine you will already be eating in a way that is conducive to avoiding or reversing type 2 diabetes, but for anyone who wants a refresher,
See: How To Prevent And Reverse Type 2 Diabetes
…which yes, as well as meaning eating/avoiding certain foods, does recommend intermittent fasting. For anyone who wants a primer on that,
See: Intermittent Fasting: Methods & Benefits
There are also drugs you may want to consider:
Metformin Without Diabetes, For Weight-Loss & More
And “nutraceuticals” that sound like drugs, for example:
Glutathione’s Benefits: The Usual And The Unique ← the good news is, it’s found in several common foods
You may have heard the hype about “nature’s Ozempic”, and berberine isn’t exactly that (works in mostly different ways), but its benefits do include improving insulin sensitivity:
Berberine For Metabolic Health
Lastly, while eating for blood sugar management is all well and good, do be aware that some things affect insulin levels without increased blood sugar levels. So even if you’re using a CGM, you may go blissfully unaware of an insulin spike, because there was no glucose spike on the graph—and in contrast, there could even be a dip in blood sugar levels, if you consumed something that increased insulin levels without providing glucose at the same time, making you think “I should have some carbs”, which visually on the graph would even out your blood sugars, but invisibly, would worsen the already-extant insulin spike.
Read more about this: Strange Things Happening In The Islets Of Langerhans: When Carbs, Proteins, & Fats Switch Metabolic Roles
Now, since you probably can’t test your insulin at a moment’s notice, the way to watch out for this is “hmm, I ate some protein/fats (delete as applicable) without carbs and my blood sugars dipped; I know what’s going on here”.
Want to know more?
We heartily recommend the “Why We Get Sick” book we linked above, as this focuses on insulin resistance/sensitivity itself!
However, a very good general primer on blood sugar management (and thus, by extension, at least moderately good insulin management), is:
Glucose Revolution: The Life-Changing Power of Balancing Your Blood Sugar – by Jessie Inchauspé
Enjoy!
Share This Post
-
Green Curry Salmon Burgers
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
These lean and healthy burgers are as quick and easy to make as they are good for entertaining. The serving-bed has its nutritional secrets too! All in all, an especially heart-healthy and brain-healthy dish.
You will need
- 4 skinless salmon fillets, cubed (Vegetarian/Vegan? Consider this Plant-Based Salmon Recipe or, since they are getting blended, simply substitute 1½ cups cooked chickpeas instead with 1 tbsp tahini)
- 2 cloves garlic, chopped
- 2 tbsp thai green curry paste
- juice of two limes, plus wedges to serve
- 1 cup quinoa
- ½ cup edamame beans, thawed if they were frozen
- large bunch fresh cilantro (or parsley if you have the “soap “cilantro tastes like soap” gene), chopped
- extra virgin olive oil, for frying
- 1 tbsp chia seeds
- 1 tbsp nutritional yeast
- 2 tsp black pepper, coarse ground
Method
(we suggest you read everything at least once before doing anything)
1) Put the salmon, garlic, curry paste, nutritional yeast, and half the lime juice into a food processor, and blend until smooth.
2) Remove, divide into four parts, and shape into burger patty shapes. Put them in the fridge where they can firm up while we do the next bit.
3) Cook the quinoa with the tablespoon of chia seeds added (which means boiling water and then letting it simmer for 10–15 minutes; when the quinoa is tender and unfurled a little, it’s done).
4) Drain the quinoa with a sieve, and stir in the edamame beans, the rest of the lime juice, the cilantro, and the black pepper. Set aside.
5) Using the olive oil, fry the salmon burgers for about 5 minutes on each side.
6) Serve; we recommend putting the burgers atop the rest, and adding a dash of lime at the table.
(it can also be served this way!)
Enjoy!
Want to learn more?
For those interested in some of the science of what we have going on today:
- Farmed Fish vs Wild–Caught
- Level-Up Your Fiber Intake! (Without Difficulty Or Discomfort)
- What Omega-3 Fatty Acids Really Do For Us
- If You’re Not Taking Chia, You’re Missing Out
- Our Top 5 Spices: How Much Is Enough For Benefits?
Take care!
Share This Post
Related Posts
-
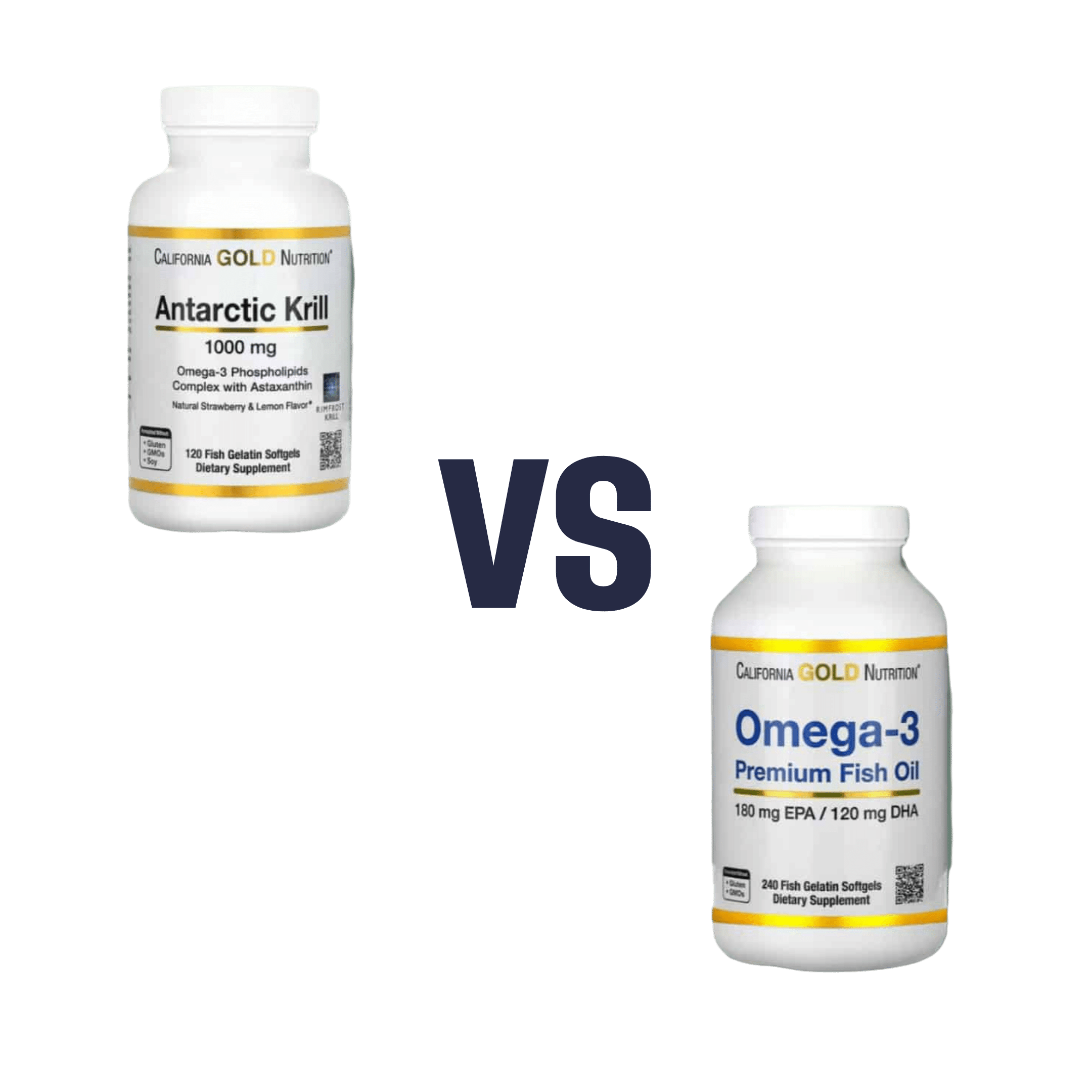
Krill Oil vs Fish Oil – Which is Healthier?
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
Our Verdict
When comparing krill oil to fish oil, we picked the krill oil.
Why?
Both of these products are good sources of omega-3 fatty acids EPA and DHA, and for the specific brand depicted above, in both cases 2 softgels will give you the recommended daily amount (which is generally held to be 250–500mg combined omega-3s per day).
This brand’s fish oil gives more (640mg combined omega-3s per 2 softgels, to the same brand’s krill oil’s 480mg per 2 softgels), but since the krill oil is already in the high end of RDA territory, the excess beyond the RDA is not helpful, and not a huge factor. More quantity is not always better, when the body can only process so much at a time.
However, the krill oil gives some extra things that the fish oil doesn’t:
- Astaxanthin, a “super-antioxidant”
- and neuroprotectant, heart-healthy phospholipids
Additional considerations
We have declared “the winner” based on health considerations only. That’s a sticking point for us in all our writings; we’ll occasionally look at and mention other factors, but we know that health is what you’re here for, so that’s what we’ll always treat as most critical.
However, in case these factors may interest you and/or influence you to one or the other:
• The fish oil is about 30% cheaper financially
• The krill oil is a lot more sustainable environmentallyBack to the health science…
Read more:
• What Omega-3 Fatty Acids Really Do For Us
• Astaxanthin: Super-Antioxidant & NeuroprotectantWant some? Here for your convenience are some example products on Amazon:
(brands available will vary per region, but now you know what to look out for on the labels!)
Don’t Forget…
Did you arrive here from our newsletter? Don’t forget to return to the email to continue learning!
Learn to Age Gracefully
Join the 98k+ American women taking control of their health & aging with our 100% free (and fun!) daily emails:
-
Rise And (Really) Shine!
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
Q&A with 10almonds Subscribers!
Q: Would love to hear more ideas about effective first thing in the morning time management to get a great start on your day.
A: There are a lot of schools of thought about what’s best in this regard! Maybe we’ll do a main feature sometime. But some things that are almost universally agreed upon are:
- Prepare your to-do list the night before
- Have some sort of buffer between waking up and getting to productivity.
- For me (hi, your writer here) it’s my first coffee of the day. It’s not even about the caffeine, it’s about the ritual of it, it’s a marker that separates my night from the day and tells my brain what gear to get into.
- Others may like to exercise first thing in the morning
- For still yet others, it could be a shower, cold or otherwise
- Some people like a tall glass of lemon water to rehydrate after sleeping!
- If you take drinkable morning supplements such as this pretty awesome nootropic stack, it’s a great time for that and an excellent way to get the brain-juices flowing!
- When you do get to productivity: eat the frog first! What this means is: if eating a frog is the hardest thing you’ll have to do all day, do that first. Basically, tackle the most intimidating task first. That way, you won’t spend your day stressed/anxious and/or subconsciously wasting time in order to procrastinate and avoid it.
- Counterpart to the above: a great idea is to also plan something to look forward to when your working day is done. It doesn’t matter much what it is, provided it’s rewarding to you, that makes you keen to finish your tasks to get to it.
Have a question you’d like to see answered here? Hit reply to this email, or use the feedback widget at the bottom! We always love to hear from you
Don’t Forget…
Did you arrive here from our newsletter? Don’t forget to return to the email to continue learning!
Learn to Age Gracefully
Join the 98k+ American women taking control of their health & aging with our 100% free (and fun!) daily emails:
-
Most People Try The Wrong Way To Unshrimp Their Posture (Here’s How To Do It Better)
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
Many people try to correct posture by pulling the shoulders back and tucking in the chin, but that doesn’t work. Happily, there is a way that does! Kinesiologist Kyle Waugh demonstrates:
Defying gravity
The trick is simple, and is about how maintaining good posture needs to be unconscious and natural, not forced. After all, who is maintaining singular focus for 16 waking hours a day?
Instead, pay attention to how the body relates to gravity without excessive muscle tension, aligning the (oft-forgotten!) hips, and maintaining balance. The importance of hip position is really not to be underestimated, since in many ways the hips are a central axis of the body just as the spine is, and the spine itself sits in the hips.
A lot of what holds the body in poor posture tends to be localized muscle tensions, so address those with stretches and relaxation exercises.
For a few quick tests and exercises to try, enjoy:
Click Here If The Embedded Video Doesn’t Load Automatically!
Want to learn more?
You might also like to read:
6 Ways To Look After Your Back ← no video on this one, just 6 concepts that you can apply to your daily life
Take care!
Don’t Forget…
Did you arrive here from our newsletter? Don’t forget to return to the email to continue learning!
Learn to Age Gracefully
Join the 98k+ American women taking control of their health & aging with our 100% free (and fun!) daily emails: