How To Rebuild Your Neurons’ Myelin Sheaths
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
PS: We Love You
Phosphatidylserine, or “PS” for short, is a phospholipid found in the brain. In other words, a kind of fatty compound that is such stuff as our brains are made of.
In particular, it’s required for healthy nerve cell membranes and myelin (the protective sheath that neurons live in—basically, myelin sheaths do for neurons what telomere caps do for DNA).
For an overview that’s more comprehensive than we have room for here, check out:
Phosphatidylserine and the human brain
Many people take it as a supplement.
Does taking it as a supplement work?
This is a valid question, as a lot of supplements can’t be absorbed well, and/or can’t pass the blood-brain barrier. But, as the above-linked study notes:
❝Exogenous PS (300-800 mg/d) is absorbed efficiently in humans, crosses the blood-brain barrier, and safely slows, halts, or reverses biochemical alterations and structural deterioration in nerve cells. It supports human cognitive functions, including the formation of short-term memory, the consolidation of long-term memory, the ability to create new memories, the ability to retrieve memories, the ability to learn and recall information, the ability to focus attention and concentrate, the ability to reason and solve problems, language skills, and the ability to communicate. It also supports locomotor functions, especially rapid reactions and reflexes.❞
(“Exogenous” means “coming from outside of the body”, as opposed to “endogenous”, meaning “made inside the body”. Effectively, in this context “exogenous” means “taken as a supplement”.)
Why do people take it?
The health claims for phosphatidylserine fall into two main categories:
- Neuroprotection (helping your brain to avoid age-related decline in the long term)
- Cognitive enhancement (helping your brain work better in the short term)
What does the science say?
There’s a lot of science that’s been done on the neuroprotective properties of PS, and there are thousands of studies we could draw from here. The upshot is that regular phosphatidylserine supplementation (most often 300mg/day, but studies are also found for 100–500mg/day) is strongly associated with a reduction in cognitive decline over the course of 12 weeks (a common study duration). Here are a some spotlight studies showing this:
- Effects of phosphatidylserine in Alzheimer’s disease
- Double-blind cross-over study of phosphatidylserine vs. placebo in patients with early dementia of the Alzheimer type
- Effect of Phosphatidylserine on Cerebral Glucose Metabolism in Alzheimer’s Disease
- The effect of soybean-derived phosphatidylserine on cognitive performance in elderly with subjective memory complaints
Note: PS can be derived from various sources, with the two most common forms being bovine (i.e., from cow brains) or soy-derived.
There is no established difference in the efficacy of these.
There have been some concerns raised about the risk of CJD (the human form of BSE, as in “mad cow disease”) from consuming brain matter from cows, but studies have not found any evidence of this actually happening.
There is also some evidence that phosphatidyserine significantly boosts cognitive performance, even in young people with no extant cognitive decline, for example:
(as the title suggests, they did also test for its effect on mood and endocrine response, but found it made no difference to those, just the cognitive function—which enjoyed a boost before exercise, as well as after it, meaning that the boost wasn’t dependent on the exercise)
PS for cognitive enhancement in the young and healthy is not nearly so well-explored as its use as a later-life guard against age-related cognitive decline. However, just because the studies in younger people are dwarfed in number by the studies in older people, doesn’t detract from the validity of the studies in younger people.
Basically: its use in older people has been studied the most, but all available evidence points to it being beneficial to brain health at all ages.
Where can we get it?
We don’t sell it (or anything else), but for your convenience, here’s an example product on Amazon.
Enjoy!
Don’t Forget…
Did you arrive here from our newsletter? Don’t forget to return to the email to continue learning!
Recommended
Learn to Age Gracefully
Join the 98k+ American women taking control of their health & aging with our 100% free (and fun!) daily emails:
-
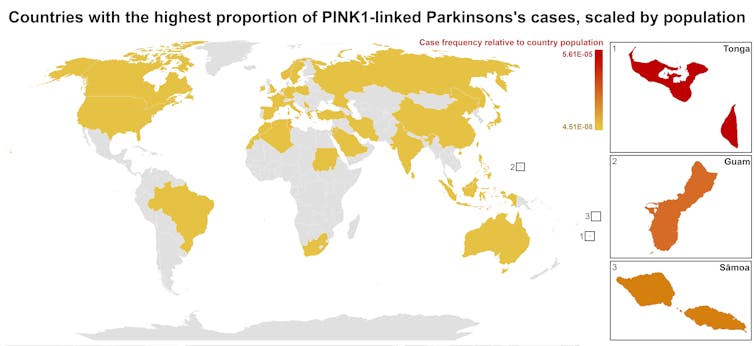
Studies of Parkinson’s disease have long overlooked Pacific populations – our work shows why that must change
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
A form of Parkinson’s disease caused by mutations in a gene known as PINK1 has long been labelled rare. But our research shows it’s anything but – at least for some populations.
Our meta-analysis revealed that people in specific Polynesian communities have a much higher rate of PINK1-linked Parkinson’s than expected. This finding reshapes not only our understanding of who is most at risk, but also how soon symptoms may appear and what that might mean for treatment and testing.
Parkinson’s disease is often thought of as a single condition. In reality, it is better understood as a group of syndromes caused by different factors – genetic, environmental or a combination of both.
These varying causes lead to differences in disease patterns, progression and subsequent diagnosis. Recognising this distinction is crucial as it paves the way for targeted interventions and may even help prevent the disease altogether.
Shutterstock/sfam_photo Why we focus on PINK1-linked Parkinson’s
We became interested in this gene after a 2021 study highlighted five people of Samoan and Tongan descent living in New Zealand who shared the same PINK1 mutation.
Previously, this mutation had been spotted only in a few more distant places –Malaysia, Guam and the Philippines. The fact it appeared in people from Samoan and Tongan backgrounds suggested a historical connection dating back to early Polynesian migrations.
One person in 1,300 West Polynesians carries this mutation. This is a frequency well above what scientists usually classify as rare (below one in 2,200). This discovery means we may be overlooking entire communities in Parkinson’s research if we continue to assume PINK1-linked cases are uncommon.
This world map shows people in some Polynesian communities have a much higher rate of PINK1-linked Parkinson’s than the global population. Eden Yin, CC BY-SA Traditional understanding says PINK1-linked Parkinson’s is both rare and typically strikes younger people, mostly in their 30s or 40s, if they inherit two faulty copies of the gene. In other words, it’s considered a recessive condition, needing two matching puzzle pieces before the disease can unfold.
Our work challenges this view. We show that even one defective PINK1 gene can cause Parkinson’s at an average age of 43, much earlier than the typical onset after 65. That’s a significant departure from the standard belief that only people with two defective gene copies are at risk.
Why this matters for people with the disease
It’s not just genetics that challenge long-held views. Historically, PINK1-linked Parkinson’s was thought to lack some of the classic features of the disease, such as toxic clumps of alpha-synuclein protein.
In typical Parkinson’s, alpha-synuclein builds up in the brain, forming sticky clumps known as Lewy bodies. Our results, contrary to prior beliefs, show that alpha-synuclein pathology is present in 87.5% of PINK1 cases. This finding opens up a promising new avenue for future treatment development.
The biggest concern is early onset. PINK1-linked Parkinson’s can begin as early as 11 years old, although a more common starting point is around the mid-30s. This early onset means living longer with the disease, which can profoundly affect education, work opportunities and family life.
Current treatments (such as levodopa, a precursor of dopamine) help manage symptoms, but they’re not designed to address the root cause. If we know someone has a PINK1 mutation, scientists and clinicians can explore therapies for specific genetic pathways, potentially delivering relief beyond symptom management.
Sex differences add a layer of complexity
In Parkinson’s, generally, men are at higher risk and tend to develop symptoms earlier. However, our findings suggest the opposite pattern for PINK1-linked cases. Particularly, women with two defective copies of the gene experience onset earlier than men.
This highlights the need to consider sex-related factors in Parkinson’s research. Overlooking them risks missing key elements of the disease.
Genetic testing could be a game-changer for PINK1-linked Parkinson’s. Because it often appears earlier, doctors may not recognise it immediately, especially if they are more familiar with the common, later-onset form of Parkinson’s.
Early genetic testing could lead to a faster, more accurate diagnosis, allowing treatment to begin when interventions are most effective. It would help families understand how the disease is inherited, enabling relatives to get tested.
In some cases, where appropriate and culturally acceptable, embryo screening may be considered to prevent the passing of the faulty gene.
Knowing you have a PINK1 mutation could also make finding the right treatment more efficient. Instead of a lengthy trial-and-error process with different medications, doctors could use emerging therapies designed to target the underlying PINK1 mutation rather than relying on general Parkinson’s treatments meant for the broader population.
Addressing research gaps
These findings underscore how crucial it is to include diverse populations in health research.
Many communities, such as those in Samoa, Tonga and other Pacific nations, have had little to no involvement in global Parkinson’s genetics studies. This has created gaps in knowledge and real-world consequences for people who may not receive timely or accurate diagnoses.
Researchers, funding bodies and policymakers must prioritise projects beyond the usual focus on European or industrialised countries to ensure research findings and treatments are relevant to all affected populations.
To better diagnose and treat Parkinson’s, we need a more inclusive approach. Recognising that PINK1-linked Parkinson’s is not as rare as previously thought – and that genetics, sex differences and cultural factors all play a role – allows us to improve care for everyone.
By expanding genetic testing, refining treatments and ensuring research reflects the full spectrum of Parkinson’s, we can move closer to more precise diagnoses, targeted therapies and better support systems for all.
Victor Dieriks, Research Fellow in Health Sciences, University of Auckland, Waipapa Taumata Rau and Eden Paige Yin, PhD candidate in Health Sciences, University of Auckland, Waipapa Taumata Rau
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Share This Post
-
The Longevity Project – by Dr. Howard Friedman & Dr. Leslie Martin
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
Most books on the topic of longevity focus on such things as diet and exercise, and indeed, those are of course important things. But what of psychological and sociological factors?
Dr. Friedman and Dr. Martin look at a landmark longitudinal study, following a large group of subjects from childhood into old age. Looking at many lifestyle factors and life events, they crunched the numbers to see what things really made the biggest impact on healthy longevity.
A strength of the book is that this study had a huge amount of data—a limitation of the book is that it often avoids giving that concrete data, preferring to say “many”, “a majority”, “a large minority”, “some”, and so forth.
However, the conclusions from the data seem clear, and include many observations such as:
- conscientiousness is a characteristic that not only promotes healthy long life, but also can be acquired as time goes by (some “carefree” children became “conscientious” adults)
- resilience is a characteristic that promotes healthy long life—but tends to only be “unlocked” by adversity
- men tend to live longer if married—women, not so much
- religion and spirituality are not big factors in healthy longevity—but social connections (that may or may not come with such) do make a big difference
Bottom line: if you’d like to know which of your decisions are affecting your healthy longevity (beyond the obvious diet, exercise, etc), this is a great book for collating that information and presenting, in essence, a guideline for a long healthy life.
Click here to check out The Longevity Project and see how it applies to your life!
Share This Post
-
How to Be Your Own Therapist – by Owen O’Kane
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
Finding the right therapist can be hard. Sometimes, even just accessing a therapist, any therapist, can be hard, if circumstances are adverse. Sometimes we’d like therapy, but want to feel “better prepared for it” before we do.
Owen O’Kane, a highly qualified and well-respected psychotherapist, wants to put some tools in our hands. The premise of this book is that “in 10 minutes a day” one can give oneself an amount of therapy that will be beneficial.
Naturally, in 10 minutes a day, this isn’t going to be the kind of therapy that will work through major traumas, so what can it do?
Those 10 minutes are spread into three sessions:
- 4 minutes in the morning
- 3 minutes in the afternoon
- 3 minutes in the evening
The idea is:
- To do a quick mental health “check-in” before the day gets started, ascertain what one needs in that context, and make a simple plan to get/have it.
- To keep one’s mental health on track by taking a little pause to reassess and adjust if necessary
- To reflect on the day, amplify the positive, and let go of the negative to what extent is practical, in order to rest well ready for the next day
Where O’Kane excels is in explaining how to do those things in a way that is neither overly simplistic and wishy-washy, nor so arcane and convoluted as to create more work and render the day more difficult.
In short, this book is a great prelude to (or adjunct to) formal therapy, and for those for whom therapy isn’t accessible and/or desired, a great way to keep oneself on a mentally healthy track.
Share This Post
Related Posts
-
Kiwi vs Lime – Which is Healthier?
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
Our Verdict
When comparing kiwi to lime, we picked the kiwi.
Why?
Looking at the macros first, kiwi has more protein, more carbs, and more fiber. As with most fruits, the fiber is the number we’re most interested in for health purposes; in this case, kiwi is just slightly ahead of lime on all three of those.
In terms of vitamins, kiwi has more of vitamins A, B2, B3, B6, B9, C, E, K, and choline, while lime has a tiny bit more vitamin B5. As in, the vitamin that’s in pretty much anything and is practically impossible to be deficient in unless you are literally starving to death. You may be thinking: aren’t limes a famously good source of vitamin C? And yes, yes they are. But kiwis have >3x more. In other big differences, kiwis also have >6x more vitamin E and >67 times more vitamin K.
When it comes to minerals, kiwi has more calcium, copper, magnesium, manganese, phosphorus, potassium, and zinc, while lime has more iron and selenium. Another easy win for kiwis.
In short: enjoy both; both are good. But kiwis are the more nutritionally dense option by almost every way of measuring it.
Want to learn more?
You might like to read:
Top 8 Fruits That Prevent & Kill Cancer ← kiwi is top of the list; it promotes cancer cell death while sparing healthy cells
Take care!
Don’t Forget…
Did you arrive here from our newsletter? Don’t forget to return to the email to continue learning!
Learn to Age Gracefully
Join the 98k+ American women taking control of their health & aging with our 100% free (and fun!) daily emails:
-
The Two-Second Advantage – by Vivek Ranadive and Kevin Maney
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
The titular “two-second advantage” can in some cases be literal (imagine you got a two-second head-start in a boxing match!), in other cases can refer to being just a little ahead of things in a way that can confer a great advantage, often cumulatively—as anyone who’s played Monopoly can certainly attest.
Vivek Ranadivé and Kevin Maney give us lots of examples from business, sports, politics, economics, and more, in a way that seeks to cultivate a habit of asking the right questions in order to anticipate the future and not just be ahead of the competition—some areas of life don’t have competition for most people, like health, for example—but to generally have things “in hand”.
When it comes to personal finances, health, personal projects, and the like, those tiny initial advantages that lead to incremental further improvements, can be the difference between continually (and frantically) playing catch-up, or making the jump past breaking even to going from strength to strength.
Don’t Forget…
Did you arrive here from our newsletter? Don’t forget to return to the email to continue learning!
Learn to Age Gracefully
Join the 98k+ American women taking control of their health & aging with our 100% free (and fun!) daily emails:
-
Led by RFK Jr., Conservatives Embrace Raw Milk. Regulators Say It’s Dangerous.
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
In summertime, cows wait under a canopy to be milked at Mark McAfee’s farm in Fresno, California. From his Cessna 210 Centurion propeller plane, the 63-year-old can view grazing lands of the dairy company he runs that produces products such as unpasteurized milk and cheese for almost 2,000 stores.
Federal regulators say it’s risky business. Samples of raw milk can contain bird flu virus and other pathogens linked to kidney disease, miscarriages, and death.
McAfee, founder and CEO of the Raw Farm, who also leads the Raw Milk Institute, says he plans to soon be in a position to change that message.
Robert F. Kennedy Jr., the anti-vaccine activist President Donald Trump has tapped to run the Department of Health and Human Services, recruited McAfee to apply for a job as the FDA’s raw milk standards and policy adviser, McAfee said. McAfee has already written draft proposals for possible federal certification of raw dairy farms, he said.
Virologists are alarmed. The Centers for Disease Control and Prevention recommends against unpasteurized dairy that hasn’t been heated to kill pathogens such as bird flu. Interstate raw milk sales for human consumption are banned by the FDA. A Trump administration that weakens the ban or extols raw milk, the scientists say, could lead to more foodborne illness. It could also, they say, raise the risk of the highly pathogenic H5N1 bird flu virus evolving to spread more efficiently, including between people, possibly fueling a pandemic.
“If the FDA says raw milk is now legal and the CDC comes through and says it advises drinking raw milk, that’s a recipe for mass infection,” said Angela Rasmussen, a virologist and co-editor-in-chief of the medical journal Vaccine and an adjunct professor at Stony Brook University in New York.
The raw milk controversy reflects the broader tensions President Donald Trump will confront when pursuing his second-administration agenda of rolling back regulations and injecting more consumer choice into health care.
Many policies Kennedy has said he wants to revisit — from the fluoridation of tap water to nutrition guidance to childhood vaccine requirements — are backed by scientific research and were established to protect public health. Some physician groups and Democrats are gearing up to fight initiatives they say would put people at risk.
Raw milk has gained a following among anti-regulatory conservatives who are part of a burgeoning health freedom movement.
“The health freedom movement was adopted by the tea party, and conspiracy websites gave it momentum,” said Paul Offit, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, who has studied the history of the anti-vaccine movement.
Once-fringe ideas are edging into the mainstream. Vaccine hesitancy is growing.
Arkansas, Utah, and Kentucky are weighing legislation that would relax or end requirements for fluoride in public water. And 30 states now allow for the sale of raw milk in some form within their borders.
While only an estimated 3% of the U.S. population consumes raw milk or cheese, efforts to try to restrict its sales have riled Republicans and provided grist for conservative podcasts.
Many conservatives denounced last year’s execution of a search warrant when Pennsylvania agriculture officials and state troopers arrived at an organic farm tucked off a two-lane road on Jan. 4, 2024. State inspectors were investigating cases of two children sickened by E. coli bacteria and sales of raw dairy from the operation owned by Amish farmer Amos Miller, according to a complaint filed by the state’s agricultural department.
Bundled in flannel shirts and winter jackets, the inspectors put orange stickers on products detaining them from sale, and they left toting product samples in large blue-and-white coolers, online videos show. The 2024 complaint against Miller alleged that he and his wife sold dairy products in violation of state law.
The farm was well known to regulators. They say in the complaint that a Florida consumer died after being sickened in 2014 with listeria bacteria found in raw dairy from Miller’s farm. The FDA said a raw milk sample from the farm indicates it was the “likely source” of the infection, based on the complaint.
Neither Miller’s farm nor his lawyer returned calls seeking comment.
The Millers’ attorney filed a preliminary objection that said “shutting down Defendants would cause inequitable harm, exceed the authority of the agency, constitute an excessive fine as well as disparate, discriminatory punishment, and contravene every essential Constitutional protection and powers reserved to the people of Pennsylvania.”
Regulators in Pennsylvania said in a press release they must protect the public, and especially children, from harm. “We cannot ignore the illnesses and further potential harm posed by distribution of these unregulated products,” the Pennsylvania agricultural department and attorney general said in a joint statement.
Unpasteurized dairy products are responsible for almost all the estimated 761 illnesses and 22 hospitalizations in the U.S. that occur annually because of dairy-related illness, according to a study published in the June 2017 issue of Emerging Infectious Diseases.
But conservatives say raiding an Amish farm is government overreach. They’re “harassing him and trying to make an example of him. Our government is really out of control,” Pennsylvania Republican Sen. Doug Mastriano said in a video he posted to Facebook.
Videos show protesters at a February 2024 hearing on Miller’s case included Amish men dressed in black with straw hats and locals waving homemade signs with slogans such as “FDA Go Away.” A court in March issued a preliminary injunction that barred Miller from marketing and selling raw dairy products within the commonwealth pending appeal, but the order did not preclude sales of raw milk to customers out of state. The case is ongoing.
With Kennedy, the raw milk debate is poised to go national. Kennedy wrote on X in October that the “FDA’s war on public health is about to end.” In the post, he pointed to the agency’s “aggressive suppression” of raw milk, as one example.
McAfee is ready. He wants to see a national raw milk ordinance, similar to one that exists for pasteurized milk, that would set minimal national standards. Farmers could attain certification through training, continuing education, and on-site pathogen testing, with one standard for farms that sell to consumers and another for retail sales.
The Trump administration didn’t return emails seeking comment.
McAfee has detailed the system he developed to ensure his raw dairy products are safe. He confirmed the process for KFF Health News: cows with yellow-tagged ears graze on grass pastures and are cleansed in washing pens before milking. The raw dairy is held back from consumer sale until it’s been tested and found clear of pathogens.
His raw dairy products, such as cheese and milk, are sold by a variety of stores, including health, organic, and natural grocery chains, according to the company website, as well as raw dairy pet products, which are not for human consumption.
He said he doesn’t believe the raw milk he sells could contain or transmit viable bird flu virus. He also said he doesn’t believe regulators’ warnings about raw milk and the virus.
“The pharmaceutical industry is trying to create a new pandemic from bird flu to get their stock back up,” said McAfee, who says he counts Kennedy as a customer. His view is not shared by leading virologists.
In December, the state of California secured a voluntary recall of all his company’s raw milk and cream products due to possible bird flu contamination.
Five indoor cats in the same household died or were euthanized in December after drinking raw milk from McAfee’s farm, and tests on four of the animals found they were infected with bird flu, according to the Los Angeles County Department of Health.
In an unrelated case, Joseph Journell, 56, said three of his four indoor cats drank McAfee’s raw milk. Two fell sick and died, he said. His third cat, a large tabby rescue named Big Boy, temporarily lost the use of his hind legs and had to use a specialized wheelchair device, he said. Urine samples from Big Boy were positive for bird flu, according to a copy of the results from Cornell University and the U.S. Department of Agriculture.
McAfee dismissed connections between the cats’ illnesses and his products, saying any potential bird flu virus would no longer be viable by the time his raw milk gets to stores. He also said he believes that any sick cats got bird flu from recalled pet food.
Journell said he has hired a lawyer to try to recover his veterinary costs but remains a staunch proponent of raw milk.
“Raw milk is good for you, just not if it has bird flu in it,” he said. “I do believe in its healing powers.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Subscribe to KFF Health News’ free Morning Briefing.
This article first appeared on KFF Health News and is republished here under a Creative Commons license.
Don’t Forget…
Did you arrive here from our newsletter? Don’t forget to return to the email to continue learning!
Learn to Age Gracefully
Join the 98k+ American women taking control of their health & aging with our 100% free (and fun!) daily emails: