Is it OK if my child eats lots of fruit but no vegetables?
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
Does it seem like most vegetables you serve your children end up left on the plate, or worse, strewn across the floor? But mention dessert, and your fruit skewers are polished off in an instant.
Or maybe the carrot and cucumber sticks keep coming home in your child’s lunchbox untouched, yet the orange slices are nowhere to be seen.
If you’re facing these struggles with your child, you’re not alone. Many children prefer fruit to vegetables.
So if your child eats lots of fruit but minimal or no vegetables, is that OK? And how can you get them to eat more veggies?
Children have an innate preference for fruit
The Australian Dietary Guidelines’ recommended daily intakes for vegetables and fruit depend on a child’s age.
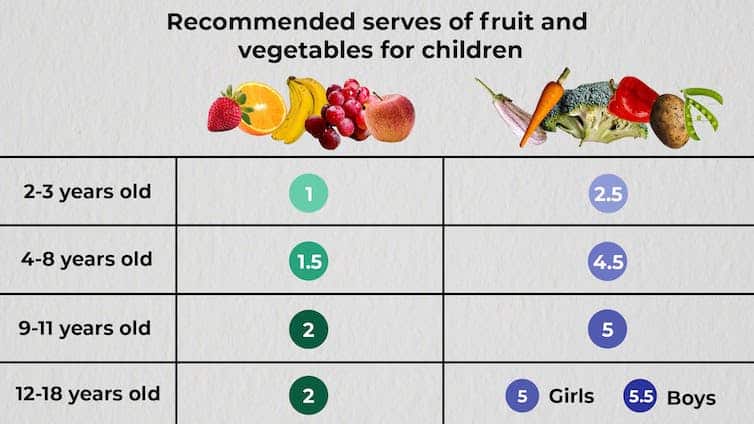
National Health and Medical Research Council, CC BY-SA
Consumption among Australian children falls well below recommendations. Around 62.6% of children aged over two meet the recommended daily fruit intake, but only 9% meet the recommended vegetable intake.
This is not surprising given children have a natural preference for fruit. At least in part, this is due to its sweetness and texture, whether crispy, crunchy or juicy. The texture of fruit has been linked to a positive sensory experience among children.
Vegetables, on the other hand, are more of an acquired taste, and certain types, such as cruciferous vegetables, can be perceived by children as bitter.
The reason children often prefer fruit over vegetables could also be related to the parents’ preferences. Some research has even suggested we develop food preferences before birth based on what our mother consumes during pregnancy.
Balance is key
So, a preference for fruit is common. But is it OK if your child eats lots of fruit but little to no vegetables? This is a question we, as dietitians, get asked regularly.
You might be thinking, at least my child is eating fruit. They could be eating no veggies and no fruit. This is true. But while it’s great your child loves fruit, vegetables are just as important as part of a balanced eating pattern.
Vegetables provide us with energy, essential vitamins and minerals, as well as water and fibre, which help keep our bowels regular. They also support a strong immune system.
If your child is only eating fruit, they are missing some essential nutrients. But the same is true if they are eating only veggies.
Fruit likewise provides the body with a variety of essential vitamins and minerals, as well as phytochemicals, which can help reduce inflammation.
Evidence shows healthy consumption of fruit and vegetables protects against chronic diseases including high blood pressure, heart disease and stroke.
Consumed together, fruit and vegetables in a variety of colours provide different nutrients we need, some of which we can’t get from other foods. We should encourage kids to eat a “rainbow” of fruit and vegetables each day to support their growth and development.
What if my child eats too much fruit?
If your child is eating slightly more fruit than what’s recommended each day, it’s not usually a problem.
Fruit contains natural sugar which is good for you. But too much of a good thing, even if it’s natural, can create problems. Fruit also contains virtually no fat and very little to no protein, both essential for a growing child.
When overindulging in fruit starts to displace other food groups such as vegetables, dairy products and meat, that’s when things can get tricky.
6 tips to get your kids to love vegetables
1. Get them involved
Take your child with you when you go shopping. Let them choose new vegetables. See if you can find vegetables even you haven’t tried, so you’re both having a new experience. Then ask them to help you with preparing or cooking the vegetables using a recipe you have chosen together. This will expose your child to veggies in a positive way and encourage them to eat more.
2. Sensory learning
Try to expose your child to vegetables rather than hiding them. Kids are more likely to eat veggies when they see, smell and feel them. This is called sensory learning.
3. Have fun with food
Use colourful vegetables of different sizes and textures. Make them fun by creating scenes or faces on your child’s plate. Add edible flowers or mint for decoration. You can even serve this with a side of veggie-based dip such as hummus or guacamole for some bonus healthy fats.
4. Teach them to grow their own
Teach your child how to grow their own vegetables. Evidence shows kids are more inclined to try the food they have helped and watched grow. You don’t need to have a big backyard to do this. A windowsill with a pot plant is a perfect start.
5. Lead by example
Your child learns from you, and your eating habits will influence theirs. Ensure they see you eating and enjoying veggies, whether in meals or as snacks.
6. Practise persistence
If your child refuses a particular vegetable once, don’t give up. It can take many attempts to encourage children to try a new food.
Yasmine Probst, Associate Professor, School of Medical, Indigenous and Health Sciences, University of Wollongong; Olivia Wills, Accredited Practising Dietitian, PhD candidate, University of Wollongong, and Shoroog Allogmanny, Accredited Practising Dietitian, PhD candidate, University of Wollongong
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Don’t Forget…
Did you arrive here from our newsletter? Don’t forget to return to the email to continue learning!
Recommended
Learn to Age Gracefully
Join the 98k+ American women taking control of their health & aging with our 100% free (and fun!) daily emails:
-
Peony Against Inflammation & More
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
Yes, this is about the flower, especially white peony (Paeonia lactiflora), and especially the root thereof (Paeoniae radix alba). Yes, the root gets a different botanical name but we promise it is the same plant. You will also read about its active glycoside paeoniflorin, and less commonly, albiflorin (a neuroprotective glycoside present in the root).
It’s one of those herbs that has made its way out of Traditional Chinese Medicine and into labs around the world.
It can be ingested directly as food, or as a powder/capsule, or made into tea.
Anti-inflammatory
Peony suppresses inflammatory pathways, which thus reduces overall inflammation. In particular, this research review found:
❝Pharmacologically, paeoniflorin exhibits powerful anti-inflammatory and immune regulatory effects in some animal models of autoimmune diseases including Rheumatoid Arthritis (RA) and Systemic Lupus Erythematosus (SLE)❞
The reviewers also (albeit working from animal models) suggest it may be beneficial in cases of kidney disease and liver disease, along with other conditions.
Here’s a larger review, which also has studies involving humans (and in vivo studies), that found it to effectively help treat autoimmune conditions including rheumatoid arthritis and psoriasis, amongst others:
❝Modern pharmacological research on TGP is based on the traditional usage of PRA, and its folk medicinal value in the treatment of autoimmune diseases has now been verified. In particular, TGP has been developed into a formulation used clinically for the treatment of autoimmune diseases.
Based on further research on its preparation, quality control, and mechanisms of action, TGP is expected to eventually play a greater role in the treatment of autoimmune diseases. ❞
(TGP = Total Glucosides of Paeony)
Antidepressant / Anxiolytic
It also acts as a natural serotonin reuptake inhibitor (as per many pharmaceutical antidepressants), by reducing the expression of the serotonin transporter protein:
Gut Microbiota-Based Pharmacokinetics and the Antidepressant Mechanism of Paeoniflorin
(remember, most serotonin is produced in the gut)
Here’s how that played out when tested (on rats, though):
Against PMS and/or menopause symptoms
Peony is widely used in Traditional Chinese Medicine to reduce these symptoms in general. However, we couldn’t find a lot of good science for that, although it is very plausible (as the extract contains phytoestrogens and may upregulate estrogen receptors while dialling down testosterone production). Here’s the best we could find for that, and it’s a side-by-side along with licorice root:
❝Paeoniflorin, glycyrrhetic acid and glycyrrhizin decreased significantly the testosterone production but did not change that of delta 4-androstenedione and estradiol. Testosterone/delta 4-androstenedione production ratio was lowered significantly by paeoniflorin, glycyrrhetic acid and glycyrrhizin❞
Effect of paeoniflorin, glycyrrhizin and glycyrrhetic acid on ovarian androgen production
(note: that it didn’t affect estradiol levels is reasonable; it contains phytoestrogens after all, not estradiol—and in fact, if you are taking estradiol, you might want to skip this one, as its phytoestrogens could compete with your estradiol for receptors)
Want to try some?
We don’t sell it, but here for your convenience is an example product on Amazon 😎
Enjoy!
Share This Post
-
Taking A Trip Through The Evidence On Psychedelics
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
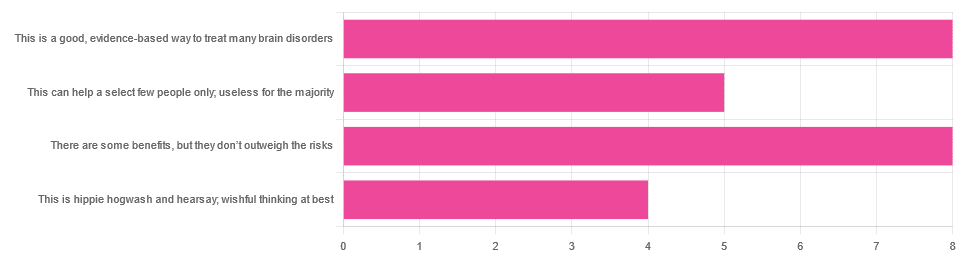
In Tuesday’s newsletter, we asked you for your opinions on the medicinal use of psychedelics, and got the above-depicted, below-described, set of responses:
- 32% said “This is a good, evidence-based way to treat many brain disorders”
- 32% said “There are some benefits, but they don’t outweigh the risks”
- 20% said “This can help a select few people only; useless for the majority”
- 16% said “This is hippie hogwash and hearsay; wishful thinking at best”
Quite a spread of answers, so what does the science say?
This is hippie hogwash and hearsay; wishful thinking at best! True or False?
False! We’re tackling this one first, because it’s easiest to answer:
There are some moderately-well established [usually moderate] clinical benefits from some psychedelics for some people.
If that sounds like a very guarded statement, it is. Part of this is because “psychedelics” is an umbrella term; perhaps we should have conducted separate polls for psilocybin, MDMA, ayahuasca, LSD, ibogaine, etc, etc.
In fact: maybe we will do separate main features for some of these, as there is a lot to say about each of them separately.
Nevertheless, looking at the spread of research as it stands for psychedelics as a category, the answers are often similar across the board, even when the benefits/risks may differ from drug to drug.
To speak in broad terms, if we were to make a research summary for each drug it would look approximately like this in each case:
- there has been research into this, but not nearly enough, as “the war on drugs” may well have manifestly been lost (the winner of the war being: drugs; still around and more plentiful than ever), but it did really cramp science for a few decades.
- the studies are often small, heterogenous (often using moderately wealthy white student-age population samples), and with a low standard of evidence (i.e. the methodology often has some holes that leave room for reasonable doubt).
- the benefits recorded are often small and transient.
- in their favor, though, the risks are also generally recorded as being quite low, assuming proper safe administration*.
*Illustrative example:
Person A takes MDMA in a club, dances their cares away, has had only alcohol to drink, sweats buckets but they don’t care because they love everyone and they see how we’re all one really and it all makes sense to them and then they pass out from heat exhaustion and dehydration and suffer kidney damage (not to mention a head injury when falling) and are hospitalized and could die;
Person B takes MDMA in a lab, is overwhelmed with a sense of joy and the clarity of how their participation in the study is helping humanity; they want to hug the researcher and express their gratitude; the researcher reminds them to drink some water.
Which is not to say that a lab is the only safe manner of administration; there are many possible setups for supervised usage sites. But it does mean that the risks are often as much environmental as they are risks inherent to the drug itself.
Others are more inherent to the drug itself, such as adverse cardiac events for some drugs (ibogaine is one that definitely needs medical supervision, for example).
For those who’d like to see numbers and clinical examples of the bullet points we gave above, here you go; this is a great (and very readable) overview:
NIH | Evidence Brief: Psychedelic Medications for Mental Health and Substance Use Disorders
Notwithstanding the word “brief” (intended in the sense of: briefing), this is not especially brief and is rather an entire book (available for free, right there!), but we do recommend reading it if you have time.
This can help a select few people only; useless for the majority: True or False?
True, technically, insofar as the evidence points to these drugs being useful for such things as depression, anxiety, PTSD, addiction, etc, and estimates of people who struggle with mental health issues in general is often cited as being 1 in 4, or 1 in 5. Of course, many people may just have moderate anxiety, or a transient period of depression, etc; many, meanwhile, have it worth.
In short: there is a very large minority of people who suffer from mental health issues that, for each issue, there may be one or more psychedelic that could help.
This is a good, evidence-based way to treat many brain disorders: True or False?
True if and only if we’re willing to accept the so far weak evidence that we discussed above. False otherwise, while the jury remains out.
One thing in its favor though is that while the evidence is weak, it’s not contradictory, insofar as the large preponderance of evidence says such therapies probably do work (there aren’t many studies that returned negative results); the evidence is just weak.
When a thousand scientists say “we’re not completely sure, but this looks like it helps; we need to do more research”, then it’s good to believe them on all counts—the positivity and the uncertainty.
This is a very different picture than we saw when looking at, say, ear candling or homeopathy (things that the evidence says simply do not work).
We haven’t been linking individual studies so far, because that book we linked above has many, and the number of studies we’d have to list would be:
n = number of kinds of psychedelic drugs x number of conditions to be treated
e.g. how does psilocybin fare for depression, eating disorders, anxiety, addiction, PTSD, this, that, the other; now how does ayahuasca fare for each of those, and so on for each drug and condition; at least 25 or 30 as a baseline number, and we don’t have that room.
But here are a few samples to finish up:
- Psilocybin as a New Approach to Treat Depression and Anxiety in the Context of Life-Threatening Diseases—A Systematic Review and Meta-Analysis of Clinical Trials
- Therapeutic Use of LSD in Psychiatry: A Systematic Review of Randomized-Controlled Clinical Trials
- Efficacy of Psychoactive Drugs for the Treatment of Posttraumatic Stress Disorder: A Systematic Review of MDMA, Ketamine, LSD and Psilocybin
- Changes in self-rumination and self-compassion mediate the effect of psychedelic experiences on decreases in depression, anxiety, and stress.
- Psychedelic Treatments for Psychiatric Disorders: A Systematic Review and Thematic Synthesis of Patient Experiences in Qualitative Studies
- Repeated lysergic acid diethylamide (LSD) reverses stress-induced anxiety-like behavior, cortical synaptogenesis deficits and serotonergic neurotransmission decline
In closing…
The general scientific consensus is presently “many of those drugs may ameliorate many of those conditions, but we need a lot more research before we can say for sure”.
On a practical level, an important take-away from this is twofold:
- drugs, even those popularly considered recreational, aren’t ontologically evil, generally do have putative merits, and have been subject to a lot of dramatization/sensationalization, especially by the US government in its famous war on drugs.
- drugs, even those popularly considered beneficial and potentially lifechangingly good, are still capable of doing great harm if mismanaged, so if putting aside “don’t do drugs” as a propaganda of the past, then please do still hold onto “don’t do drugs alone”; trained professional supervision is a must for safety.
Take care!
Share This Post
-
Hypertension: Factors Far More Relevant Than Salt
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
Hypertension: Factors Far More Relevant Than Salt
Firstly, what is high blood pressure vs normal, and what do those blood pressure readings mean?
Rather than take up undue space here, we’ll just quickly link to…
Blood Pressure Readings Explained (With A Colorful Chart)
More details of specifics, at:
Hypotension | Normal | Elevated | Stage 1 | Stage 2 | Danger zone
Keeping Blood Pressure Down
As with most health-related things (and in fact, much of life in general), prevention is better than cure.
People usually know “limit salt” and “manage stress”, but there’s a lot more to it!
Salt isn’t as big a factor as you probably think
That doesn’t mean go crazy on the salt, as it can cause a lot of other problems, including organ failure. But it does mean that you can’t skip the salt and assume your blood pressure will take care of itself.
This paper, for example, considers “high” sodium consumption to be more than 5g per day, and urinary excretion under 3g per day is considered to represent a low sodium dietary intake:
Sodium Intake and Hypertension
Meanwhile, health organizations often recommend to keep sodium intake to under 2g or under 1.5g
Top tip: if you replace your table salt with “reduced sodium” salt, this is usually sodium chloride (regular table salt) cut with potassium chloride, which is almost as “salty” tastewise, but obviously contains less sodium. Not only that, but potassium actually helps the body eliminate sodium, too.
The rest of what you eat is important too
The Mediterranean Diet is as great for this as it is for most health conditions.
If you sometimes see the DASH diet mentioned, that stands for “Dietary Approaches to Stop Hypertension”, and is basically the Mediterranean Diet with a few tweaks.
What are the tweaks?
- Beans went down a bit in priority
- Red meat got removed entirely instead of “limit to a tiny amount”
- Olive oil was deprioritized, and/but vegetable oil is at the bottom of the list (i.e., use sparingly)
You can check out the details here, with an overview and examples:
DASH Eating Plan—Description, Charts, and Recipes
Don’t drink or smoke
And no, a glass of red a day will not help your heart. Alcohol does make us feel relaxed, but that is because of what it does to our brain, not what it does to our heart.
In reality, even a single drink will increase blood pressure. Yes, really:
And smoking? It’s so bad that even second-hand smoke increases blood pressure:
Get those Zs in
Sleep is a commonly underestimated/forgotten part of health, precisely because in a way, we’re not there for it when it happens. We sleep through it! But it is important, including to protect against hypertension:
Short- and long-term health consequences of sleep disruption
Move your body!
Moving your body often is far more important for your heart than running marathons or bench-pressing your spouse.
Those 150 minutes “moderate exercise” (e.g. walking) per week are important, and can be for example:
- 22 minutes per day, 7 days per week
- 25 minutes per day, 6 days per week
- 30 minutes per day, 5 days per week
- 75 minutes per day, 2 days per week
If you’d like to know more about the science and evidence for this, as well as practical suggestions, you can download the complete second edition of the Physical Activity Guidelines for Americans here (it’s free, and no sign-up required!)
If you prefer a bite-size summary, then here’s their own:
Top 10 Things to Know About the Second Edition of the Physical Activity Guidelines for Americans
PS: Want a blood pressure monitor? We don’t sell them (or anything else), but for your convenience, here’s a good one you might want to consider.
Share This Post
Related Posts
-
ADHD 2.0 – by Dr. Edward Hallowell & Dr. John Ratey
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
A lot of ADHD literature is based on the assumption that the reader is a 30-something parent of a child with ADHD. This book, on the other hand, addresses all ages, and includes just as readily the likelihood that the person with ADHD is the reader, and/or the reader’s partner.
The authors cover such topics as:
- ADHD mythbusting, before moving on to…
- The problems of ADHD, and the benefits that those exact same traits can bring too
- How to leverage those traits to get fewer of the problems and more of the benefits
- The role of diet beyond the obvious, including supplementation
- The role of specific exercises (especially HIIT, and balance exercises) in benefiting the ADHD brain
- The role of medications—and arguments for and gainst such
The writing style is… Thematic, let’s say. The authors have ADHD and it shows. So, expect comprehensive deep-dives from whenever their hyperfocus mode kicked in, and expect no stones left unturned. That said, it is very readable, and well-indexed too, for ease of finding specific sub-topics.
Bottom line: if you are already very familiar with ADHD, you may not learn much, and might reasonably skip this one. However, if you’re new to the topic, this book is a great—and practical—primer.
Don’t Forget…
Did you arrive here from our newsletter? Don’t forget to return to the email to continue learning!
Learn to Age Gracefully
Join the 98k+ American women taking control of their health & aging with our 100% free (and fun!) daily emails:
-
How I Cured My Silent Reflux – by Don Daniels
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
Acid reflux, in its various forms (not all of which include heartburn as a symptom!), affects around 1 in 8 people. Often it takes the form of coughing or excess mucus after eating, and it can trigger ostensibly random sweats, for example.
Don Daniels does an excellent job of demystifying the various kinds of acid reflux, explaining clearly and simply the mechanics of what is going on for each of them and why.
Further, he talks about the medications that can make things worse (and how and why), and supplements that can make it better (and supplements that can make it worse, too!), and a multiphase plan (diet on, meds weaned off, supplements on, supplements weaned off when asymptomatic, diet adjust to a new normal) to get free from acid reflux.
The writing style is simple, clear, and jargon-free, while referencing plenty of scientific literature, often quoting from it and providing sources, much like we often do at 10almonds. There are 50+ such references in all, for a 105-page book.
So, do also note that yes, it’s quite a short book for the price, but the content is of value and wouldn’t have benefitted from padding of the kind that many authors do just to make the book longer.
Bottom line: if you have, or suspect you may have, an acid reflux condition of any kind, then this book can guide you through fixing that.
Click here to check out How I Cured My Silent Reflux, and put up with it no longer!
Don’t Forget…
Did you arrive here from our newsletter? Don’t forget to return to the email to continue learning!
Learn to Age Gracefully
Join the 98k+ American women taking control of their health & aging with our 100% free (and fun!) daily emails:
-
Treat Your Own Back – by Robin McKenzie
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
A quick note about the author first: he’s a physiotherapist and not a doctor, but with over 40 years of practice to his name and 33 letters after his name (CNZM OBE FCSP (Hon) FNZSP (Hon) Dip MDT Dip MT), he seems to know his stuff. And certainly, if you visit any physiotherapist, they will probably have some of his books on their own shelves.
This book is intended for the layperson, and as such, explains everything that you need to know, in order to diagnose and treat your back. To this end, he includes assorted tests to perform, a lot of details about various possible back conditions, and then exercises to fix it, i.e. fix whatever you have now learned that the problem is, in your case (if indeed you didn’t know for sure already).
Of course, not everything can be treated by exercises, and he does point to what other things may be necessary in those cases, but for the majority, a significant improvement (if not outright symptom-free status) can be enjoyed by applying the techniques described in this book.
Bottom line: for most people, this book gives you the tools required to do exactly what the title says.
Click here to check out Treat Your Own Back, and treat your own back!
PS: if your issue is not with your back, we recommend you check out his other books in the series (neck, shoulder, hip, knee, ankle) 😎
Don’t Forget…
Did you arrive here from our newsletter? Don’t forget to return to the email to continue learning!
Learn to Age Gracefully
Join the 98k+ American women taking control of their health & aging with our 100% free (and fun!) daily emails: