Better Than BMI
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
BMI is a very flawed system, and there are several more useful ways of measuring our bodies. Let’s take a look at them!
What’s wrong with BMI?
Oof, what isn’t wrong with BMI?
In short, it was developed as a demographic-based tool to specifically chart the weight-related health of working-age European white men a little under 200 years ago.
This means that if you are, perchance, not a working-age European white man in 1830 or so, then it’s not so useful. It’d be like first establishing height norms based on NBA basketball players, and then applying it to the general population, and thus coming to the conclusion that someone who is 6’2″ is very short.
In long, we did a deep-dive into it here, and in particular what things go dangerously wrong when it’s applied to women, non-white people, athletic people, pregnant people, people under 16 or over 65 and more:
When BMI Doesn’t Quite Measure Up
What we usually recommend instead
For heart disease risk and diabetes risk both, waist circumference is a much more universally reliable indicator. And since those two things tend to affect a lot of other health risks, it becomes an excellent starting point for being aware of many aspects of health.
Pregnancy will still throw off waist circumference a little (measure below the bump, not around it!), but it will nevertheless be more helpful than BMI even then, as it becomes necessary to just increase the numbers a little, according to gestational month and any confounding factors e.g. twins, triplets, etc. Ask your obstetrician about this, as it’s beyond the scope of our article today!
As to what’s considered a risk:
- Waist circumference of more than 35 inches for women
- Waist circumference of more than 40 inches for men
These numbers are considered applicable across demographics of age, ethnicity, and lifestyle.
Bonus extra measurement based on the above
Important also is waist to hip ratio.
How to calculate it:
- measure your waist circumference
- measure your hip circumference
- divide the first measurement by the second one
Because it’s a ratio, it doesn’t matter what units you use (e.g. inches, cm, etc) so long as you use the same units for both measurements.
The World Health Organization offers the following chart:
| Health risk | Women | Men |
| Low | 0.80 or lower | 0.95 or lower |
| Moderate | 0.81–0.85 | 0.96–1.0 |
| High | 0.86 or higher | 1.1 or higher |
Source: Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation
This is especially relevant for cardiovascular disease risk:
…and also holds true for all-cause mortality:
Waist-Hip-Ratio as a Predictor of All-Cause Mortality in High-Functioning Older Adults
An ancient contender that’s still more useful than BMI
Remember Archimedes? The (perhaps apocryphal) story of his “Eureka” moment in the bathtub when he realized that water displacement could be used to measure the volume of an irregular shape?
Just like Archimedes (who, the story goes, had been hired to determine the composition of a crown that might or might not have been pure gold), we can use this method to determine body composition, because we have references for how much a given volume of a given substance will weigh, so combing what we know about a body’s weight and volume will tell us about its composition in ways that neither metric could give us alone.
Indeed, it’s one of the commonly-mentioned flaws of BMI that muscle weighs more than fat, and Archimedes’ method not only avoids that problem, but also, actually turns that knowledge (muscle weighs more than fat) to our advantage.
It’s called “hydrostatic weighing” now:
You may be wondering: what about bones? Or internal organs?
The fact is that those are slightly confounding factors that do get in the way of a truly accurate analysis, but the variation in how much one person’s skeleton weighs vs another’s, or one person’s set of organs weigh than another’s, is too small to make an important difference to the health implications.
Lastly…
Hydrostatic weighing isn’t the only way to work out how much of our body is made of fat; if you have for example a smart scale at home (like this one) that tells you your body fat percentage, that is an estimate based on bioelectrical impedance analysis.
It’s less accurate than the hydrostatic method, but easier to do at home!
As to what percentages are “best”, healthy body fat percentages are (assuming normal hormones) generally considered to be in the range of 20–25% for women and 15–20% for men.
You can read more about this here:
Is A Visible Six-Pack Obtainable Regardless Of Genetic Predisposition?
Take care!
Don’t Forget…
Did you arrive here from our newsletter? Don’t forget to return to the email to continue learning!
Recommended
Learn to Age Gracefully
Join the 98k+ American women taking control of their health & aging with our 100% free (and fun!) daily emails:
-
5 Ways To Beat Cancer (And Other Diseases)
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
A Systematic Approach To Healthy Eating
This is Dr. William Li. He’s a physician, cancer researcher, and educator. He also founded the Angiogenesis Foundation back in 1994.
We recently reviewed one of his books, “Eat To Beat Disease”.
He has another book that we haven’t reviewed at time of writing, “Eat To Beat Your Diet“, which you might like to check out.
What does he want us to know?
He wants us to know how to eat to beat cancer and other diseases, by means of five specific angles:
Angiogenesis
This is about replacing blood vessels, which of course happens all the time, but it becomes a problem when it is feeding a cancer in the process.
Here, based on Dr. Li’s work, is what can be done about it:
A List of Anti-Angiogenic Foods for a Cancer-Fighting Diet
Regeneration
Generally speaking, we want to replace healthy cells early, because if we wait until they get damaged, then that damage will be copied forwards. As well as intermittent fasting, there are other things we can do to promote this—even, Dr. Li’s research shows, for stem cells:
Doctor’s Tip: Regeneration (stem cells)—one of your body’s five defense systems
Microbiome health
Healthy gut, healthy rest of the body. We’ve written about this before:
Making Friends With Your Gut (You Can Thank Us Later)
DNA protection
DNA gets unravelled and damaged with age, the telomere caps get shorter, and mistakes get copied forward. So there more we can protect our DNA, the longer we can live healthily. There are many ways to do this, but Dr. Li was one of the first to bring to light the DNA-protecting benefits of kiwi fruit:
Immunity
Paradoxically, what’s good for your immune system (making it stronger) also helps to protect against autoimmune diseases (for most people, for the most part).
In short: it’s good to have an immune system that’s powerful not just in its counterattacks, but also in its discerning nature. There are dietary and other lifestyle approaches to both, and they’re mostly the same things:
Beyond Supplements: The Real Immune-Boosters!
and thus see also:
Want to know more?
You might enjoy his blog or podcast, and here’s his TED talk:
Want to watch it, but not right now? Bookmark it for later
Enjoy!
Share This Post
-
Getting antivirals for COVID too often depends on where you live and how wealthy you are
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
Medical experts recommend antivirals for people aged 70 and older who get COVID, and for other groups at risk of severe illness and hospitalisation from COVID.
But many older Australians have missed out on antivirals after getting sick with COVID. It is yet another way the health system is failing the most vulnerable.
CGN089/Shutterstock Who missed out?
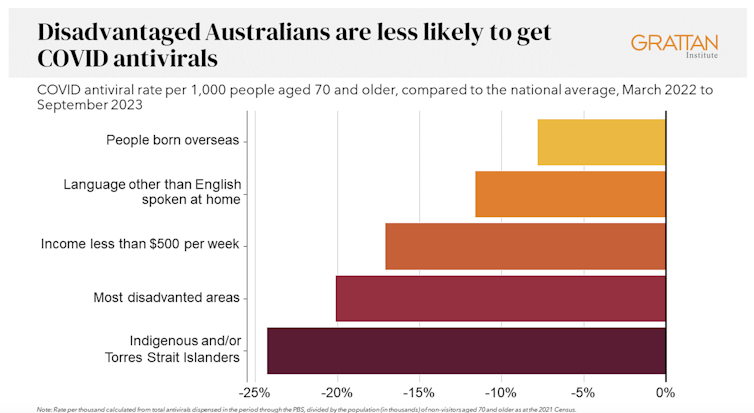
We analysed COVID antiviral uptake between March 2022 and September 2023. We found some groups were more likely to miss out on antivirals including Indigenous people, people from disadvantaged areas, and people from culturally and linguistically diverse backgrounds.
Some of the differences will be due to different rates of infection. But across this 18-month period, many older Australians were infected at least once, and rates of infection were higher in some disadvantaged communities.
How stark are the differences?
Compared to the national average, Indigenous Australians were nearly 25% less likely to get antivirals, older people living in disadvantaged areas were 20% less likely to get them, and people with a culturally or linguistically diverse background were 13% less likely to get a script.
People in remote areas were 37% less likely to get antivirals than people living in major cities. People in outer regional areas were 25% less likely.
Dispensing rates by group. Grattan Institute Even within the same city, the differences are stark. In Sydney, people older than 70 in the affluent eastern suburbs (including Vaucluse, Point Piper and Bondi) were nearly twice as likely to have had an antiviral as those in Fairfield, in Sydney’s south-west.
Older people in leafy inner-eastern Melbourne (including Canterbury, Hawthorn and Kew) were 1.8 times more likely to have had an antiviral as those in Brimbank (which includes Sunshine) in the city’s west.
Why are people missing out?
COVID antivirals should be taken when symptoms first appear. While awareness of COVID antivirals is generally strong, people often don’t realise they would benefit from the medication. They wait until symptoms get worse and it is too late.
Frequent GP visits make a big difference. Our analysis found people 70 and older who see a GP more frequently were much more likely to be dispensed a COVID antiviral.
Regular visits give an opportunity for preventive care and patient education. For example, GPs can provide high-risk patients with “COVID treatment plans” as a reminder to get tested and seek treatment as soon as they are unwell.
Difficulty seeing a GP could help explain low antiviral use in rural areas. Compared to people in major cities, people in small rural towns have about 35% fewer GPs, see their GP about half as often, and are 30% more likely to report waiting too long for an appointment.
Just like for vaccination, a GP’s focus on antivirals probably matters, as does providing care that is accessible to people from different cultural backgrounds.
Care should go those who need it
Since the period we looked at, evidence has emerged that raises doubts about how effective antivirals are, particularly for people at lower risk of severe illness. That means getting vaccinated is more important than getting antivirals.
But all Australians who are eligible for antivirals should have the same chance of getting them.
These drugs have cost more than A$1.7 billion, with the vast majority of that money coming from the federal government. While dispensing rates have fallen, more than 30,000 packs of COVID antivirals were dispensed in August, costing about $35 million.
Such a huge investment shouldn’t be leaving so many people behind. Getting treatment shouldn’t depend on your income, cultural background or where you live. Instead, care should go to those who need it the most.
Getting antivirals shouldn’t depend on who your GP is. National Cancer Institute/Unsplash People born overseas have been 40% more likely to die from COVID than those born here. Indigenous Australians have been 60% more likely to die from COVID than non-Indigenous people. And the most disadvantaged people have been 2.8 times more likely to die from COVID than those in the wealthiest areas.
All those at-risk groups have been more likely to miss out on antivirals.
It’s not just a problem with antivirals. The same groups are also disproportionately missing out on COVID vaccination, compounding their risk of severe illness. The pattern is repeated for other important preventive health care, such as cancer screening.
A 3-step plan to meet patients’ needs
The federal government should do three things to close these gaps in preventive care.
First, the government should make Primary Health Networks (PHNs) responsible for reducing them. PHNs, the regional bodies responsible for improving primary care, should share data with GPs and step in to boost uptake in communities that are missing out.
Second, the government should extend its MyMedicare reforms. MyMedicare gives general practices flexible funding to care for patients who live in residential aged care or who visit hospital frequently. That approach should be expanded to all patients, with more funding for poorer and sicker patients. That will give GP clinics time to advise patients about preventive health, including COVID vaccines and antivirals, before they get sick.
Third, team-based pharmacist prescribing should be introduced. Then pharmacists could quickly dispense antivirals for patients if they have a prior agreement with the patient’s GP. It’s an approach that would also work for medications for chronic diseases, such as cardiovascular disease.
COVID antivirals, unlike vaccines, have been keeping up with new variants without the need for updates. If a new and more harmful variant emerges, or when a new pandemic hits, governments should have these systems in place to make sure everyone who needs treatment can get it fast.
In the meantime, fairer access to care will help close the big and persistent gaps in health between different groups of Australians.
Peter Breadon, Program Director, Health and Aged Care, Grattan Institute
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Share This Post
-
Steps For Keeping Your Feet A Healthy Foundation
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
Important Steps For Good Health
This is Dr. Kelly Starrett. He’s a physiotherapist, author, speaker, trainer. He has been described as a “celebrity” and “founding father” of CrossFit. He mostly speaks and writes about mobility in general; today we’re going to be looking at what he has to say specifically about our feet.
A strong foundation
“An army marches on its stomach”, Napoleon famously wrote.
More prosaically: an army marches on its feet, and good foot-care is a top priority for soldiers—indeed, in some militaries, even so much as negligently getting blisters is a military offense.
Most of us are not soldiers, but there’s a lesson to be learned here:
Your feet are the foundation for much of the rest of your health and effectiveness.
KISS for feet
No, not like that.
Rather: “Keep It Simple, Stupid”
Dr. Starrett is not only a big fan of not overcomplicating things, but also, he tells us how overcomplicating things can actively cause problems. When it comes to footwear, for example, he advises:
❝When you wear shoes, wear the flat kind. If you’re walking the red carpet on Oscar night, fine, go ahead and wear a shoe with a heel. Once in a while is okay.
But most of the time, you should wear shoes that are flat and won’t throw your biological movement hardware into disarray.
When you have to wear shoes, whether it’s running shoes, work shoes, or combat boots, buy the flat kind, also known as “zero drop”—meaning that the heel is not raised above the forefoot (at all).
What you want to avoid, or wean yourself away from, are shoes with the heels raised higher off the ground than the forefeet.❞
Of course, going barefoot is great for this, but may not be an option for all of us when out and about. And in the home, going barefoot (or shod in just socks) will only confer health benefits if we’re actually on our feet! So… How much time do you spend on your feet at home?
Allow your feet to move like feet
By evolution, the human body is built for movement—especially walking and running. That came with moving away from hanging around in trees for fruit, to hunting and gathering between different areas of the savannah. Today, our hunting and gathering may be done at the local grocery store, but we still need to keep our mobility, especially when it comes to our feet.
Now comes the flat footwear you don’t want: flip-flops and similar
If we wear flip-flops, or other slippers or shoes that hold onto our feet only at the front, we’re no longer walking like we’re supposed to. Instead of being the elegant product of so much evolution, we’re now walking like those AT-AT walkers in Star Wars, you know, the ones that fell over so easily?
Our feet need to be able to tilt naturally while walking/running, without our footwear coming off.
Golden rule for this: if you can’t run in them, you shouldn’t be walking in them
Exception: if for example you need something on your feet for a minute or two in the shower at the gym/pool, flip-flops are fine. But anything more than that, and you want something better.
Watch your step
There’s a lot here that’s beyond the scope of what we can include in this short newsletter, but:
If we stand or walk or run incorrectly, we’re doing gradual continual damage to our feet and ankles (potentially also our knees and hips, which problems in turn have a knock-on effect for our spine, and you get the idea—this is Bad™)
Some general pointers for keeping things in good order include:
- Your weight should be mostly on the balls of your feet, not your heels
- Your feet should be pretty much parallel, not turned out or in
- When standing, your center of gravity should be balanced between heel and forefoot
Quick tip for accomplishing this last one: Stand comfortably, your feet parallel, shoulder-width apart. Now, go up on your tip-toes. When you’ve done so, note where your spine is, and keep it there (apart from in its up-down axis) when you slowly go back to having your feet flat on the ground, so it’s as though your spine is sliding down a pole that’s fixed in place.
If you do this right, your center of gravity will now be perfectly aligned with where it’s supposed to be. It might feel a bit weird at first, but you’ll get used to it, and can always reset it whenever you want/need, by repeating the exercise.
If you’d like to know more from Dr. Starrett, you can check out his website here 🙂
Share This Post
Related Posts
-
All of your hepatitis B vaccine questions answered
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
Hepatitis B is a viral infection that can cause liver disease in people of any age or background. Vaccination is 95 percent effective against the virus. But in recent years, false claims, rumors, and myths about the hepatitis B vaccine have become increasingly common.
Here’s everything you need to know about the lifesaving hepatitis B vaccine.
What is hepatitis B?
Hepatitis B is a liver infection caused by the hepatitis B virus. The virus attacks the liver, causing severe short-term and long-term infections.
Short-term hepatitis B infections may cause “fever, fatigue, loss of appetite, nausea, vomiting, jaundice (yellow skin or eyes, dark urine, clay-colored bowel movements), and pain in the muscles, joints, and stomach,” according to the Centers for Disease Control and Prevention.
A long-term hepatitis B infection occurs when the virus stays in the body beyond the initial infection, causing chronic illness. Hepatitis B infections become chronic in 90 percent of infected infants, half of infected young children, and between 5 to 10 percent of infected adults.
“Most people who go on to develop chronic hepatitis B do not have symptoms, but it is still very serious and can lead to liver damage (cirrhosis), liver cancer, and death. Chronically infected people can spread hepatitis B virus to others, even if they do not feel or look sick themselves,” says the CDC.
How does the hepatitis B virus spread?
The hepatitis B virus is spread through body fluids, including blood, semen, and saliva. It can also be transmitted from birthing parent to child during pregnancy and childbirth.
“While hepatitis B is an infection that lives in bodily fluids, it can survive outside the human body for several days, which means that sharing contaminated household products is a possible source of infection,” said Dr. Christopher Labos, a McGill University cardiologist and epidemiologist, in a 2019 article.
In 2022, over 250 million people worldwide had chronic hepatitis B, and 1.1 million died from the disease. Most of the deaths were from liver damage and liver cancer. Less than 15 percent of people living with hepatitis B have been diagnosed.
How well does the vaccine protect against hepatitis B?
Hepatitis B vaccination is up to 95 percent effective, providing lasting—and possibly lifelong—protection against the virus. Depending on when the first dose is given, the complete vaccine series consists of two to three doses.
The vaccine is most effective for infants and children. The CDC recommends that infants receive it at birth for the most protection.
The first dose is followed by two to three additional doses administered before 18 months. Children, adolescents, and adults who weren’t vaccinated as infants should also receive the vaccine.
Vaccination is particularly important for high-risk groups, including health workers and those who are in close contact with individuals living with chronic hepatitis B, people who use intravenous drugs, and people receiving blood transfusions, dialysis, or organ transplants.
Is the vaccine safe?
Vaccines against hepatitis B were first developed in the 1980s, and they have been proven safe for decades. They have a low risk of serious side effects and are safe enough to be given to newborns, pregnant people, and immunocompromised people.
We also know hepatitis B vaccines work: “Between 1990 (about the time when universal hepatitis B vaccinations started) and 2006, the rate of hepatitis B infection fell by 81 percent to the lowest level ever recorded, and the decline was greatest among children,” added Labos.
Hepatitis B rates have continued to decline across all age groups, with the U.S. exceeding its goal of reducing new hepatitis B infections by 20 percent.
Why do doctors recommend the vaccine for babies?
Hepatitis B vaccination helps protect infants from a lifetime of potentially life-threatening infections and complications. Nine out of 10 unvaccinated infants infected with hepatitis B will develop chronic infections, which increases their risk of liver failure and liver cancer.
The hepatitis B vaccine is administered at birth to help prevent the virus from being transmitted from birthing parent to child. It also helps protect infants who might be in close contact with someone with hepatitis B. This is particularly important because most people who have hepatitis are undiagnosed.
Have more questions? Talk to your health care provider to learn more about hepatitis B vaccination.
This article first appeared on Public Good News and is republished here under a Creative Commons license.
Don’t Forget…
Did you arrive here from our newsletter? Don’t forget to return to the email to continue learning!
Learn to Age Gracefully
Join the 98k+ American women taking control of their health & aging with our 100% free (and fun!) daily emails:
-
Serotonin vs Dopamine (Know The Differences)
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
Of the various neurotransmitters that people confuse with each other, serotonin and dopamine are the two highest on the list (with oxytocin coming third as people often attribute its effects to serotonin). But, for all they are both “happiness molecules”, serotonin and dopamine are quite different, and are even opposites in some ways:
More than just happiness
Let’s break it down:
Similarities:
- Both are neurotransmitters, neuromodulators, and monoamines.
- Both impact cognition, mood, energy, behavior, memory, and learning.
- Both influence social behavior, though in different ways.
Differences (settle in; there are many):
- Chemical structure:
- Dopamine: catecholamine (derived from phenylalanine and tyrosine)
- Serotonin: indoleamine (derived from tryptophan)
- Derivatives:
- Dopamine → noradrenaline and adrenaline (stress and alertness)
- Serotonin → melatonin (sleep and circadian rhythm)
- Effects on mental state:
- Dopamine: drives action, motivation, and impulsivity.
- Serotonin: promotes calmness, behavioral inhibition, and cooperation.
- Role in memory and learning:
- Dopamine: key in attention and working memory
- Serotonin: crucial for hippocampus activation and long-term memory
Symptoms of imbalance:
- Low dopamine:
- Loss of motivation, focus, emotion, and activity
- Linked to Parkinson’s disease and ADHD
- Low serotonin:
- Sadness, irritability, poor sleep, and digestive issues
- Linked to PTSD, anxiety, and OCD
- High dopamine:
- Excessive drive, impulsivity, addictions, psychosis
- High serotonin:
- Nervousness, nausea, and in extreme cases, serotonin syndrome (which can be fatal)
Brain networks:
- Dopamine: four pathways controlling movement, attention, executive function, and hormones.
- Serotonin: widely distributed across the cortex, partially overlapping with dopamine systems.
Speed of production:
- Dopamine: can spike and deplete quickly; fatigues faster with overuse.
- Serotonin: more stable, releasing steadily over longer periods.
Illustrative examples:
- Coffee boosts dopamine but loses its effect with repeated use.
- Sunlight helps maintain serotonin levels over time.
If you remember nothing else, remember this:
- Dopamine: action, motivation, and alertness.
- Serotonin: contentment, happiness, and calmness.
For more on all of the above, enjoy:
Click Here If The Embedded Video Doesn’t Load Automatically!
Want to learn more?
You might also like to read:
Take care!
Don’t Forget…
Did you arrive here from our newsletter? Don’t forget to return to the email to continue learning!
Learn to Age Gracefully
Join the 98k+ American women taking control of their health & aging with our 100% free (and fun!) daily emails:
-
Fast Diet, Fast Exercise, Fast Improvements
10almonds is reader-supported. We may, at no cost to you, receive a portion of sales if you purchase a product through a link in this article.
Diet & Exercise, Optimized
This is Dr. Michael Mosley. He originally trained in medicine with the intention of becoming a psychiatrist, but he grew disillusioned with psychiatry as it was practised, and ended up pivoting completely into being a health educator, in which field he won the British Medical Association’s Medical Journalist of the Year Award.
He also died under tragic circumstances very recently (he and his wife were vacationing in Greece, he went missing while out for a short walk on the 5th of June, appears to have got lost, and his body was found 100 yards from a restaurant on the 9th). All strength and comfort to his family; we offer our small tribute here today in his honor.
The “weekend warrior” of fasting
Dr. Mosley was an enjoyer (and proponent) of intermittent fasting, which we’ve written about before:
Fasting Without Crashing? We Sort The Science From The Hype
However, while most attention is generally given to the 16:8 method of intermittent fasting (fast for 16 hours, eat during an 8 hour window, repeat), Dr. Mosley preferred the 5:2 method (which generally means: eat at will for 5 days, then eat a reduced calorie diet for the other 2 days).
Specifically, he advocated putting that cap at 800 kcal for each of the weekend days (doesn’t have to be specifically the weekend).
He also tweaked the “eat at will for 5 days” part, to “eat as much as you like of a low-carb Mediterranean diet for 5 days”:
❝The “New 5:2” approach involves restricting calories to 800 on fasting days, then eating a healthy lower carb, Mediterranean-style diet for the rest of the week.
The beauty of intermittent fasting means that as your insulin sensitivity returns, you will feel fuller for longer on smaller portions. This is why, on non-fasting days, you do not have to count calories, just eat sensible portions. By maintaining a Mediterranean-style diet, you will consume all of the healthy fats, protein, fibre and fresh plant-based food that your body needs.❞
Read more: The Fast 800 | The New 5:2
And about that tweaked Mediterranean Diet? You might also want to check out:
Four Ways To Upgrade The Mediterranean Diet
Knowledge is power
Dr. Mosley encouraged the use of genotyping tests for personal health, not just to know about risk factors, but also to know about things such as, for example, whether you have the gene that makes you unable to gain significant improvements in aerobic fitness by following endurance training programs:
The Real Benefit Of Genetic Testing
On which note, he himself was not a fan of exercise, but recognised its importance, and instead sought to minimize the amount of exercise he needed to do, by practising High Intensity Interval Training. We reviewed a book of his (teamed up with a sports scientist) not long back; here it is:
Fast Exercise: The Simple Secret of High Intensity Training – by Dr. Michael Mosley & Peta Bee
You can also read our own article on the topic, here:
How To Do HIIT (Without Wrecking Your Body)
Just One Thing…
As well as his many educational TV shows, Dr. Mosley was also known for his radio show, “Just One Thing”, and a little while ago we reviewed his book, effectively a compilation of these:
Just One Thing: How Simple Changes Can Transform Your Life – by Dr. Michael Mosley
Enjoy!
Don’t Forget…
Did you arrive here from our newsletter? Don’t forget to return to the email to continue learning!
Learn to Age Gracefully
Join the 98k+ American women taking control of their health & aging with our 100% free (and fun!) daily emails: